Gus Mueller: It's good to be back for another edition of Vanderbilt Audiology Journal Club. All of the Vanderbilt Audiology Journal Club sessions on AudiologyOnline feature a guest from the Vanderbilt University audiology faculty reviewing and discussing pertinent journal articles around a particular theme. Today's guest is Dr. Devin McCaslin, and he will be talking about the video head impulse test. In addition, each Vandy Journal Club contains a few other special features relevant to audiology clinical practice that we hope keep these seminars lively and interesting, including What They're Reading at Vandy, Gus' Pick of the Month, and Vandy Vignettes. So let's get started.
What They're Reading at Vandy
In this segment, we feature an article or two recommended by one of our audiology team members at Vanderbilt. Today we are featuring Dr. René Gifford. René's interest primarily is in cochlear implants. She mentioned a few articles that she thought were interesting, and that is what I am going to relay to you. When it comes to cochlear implants, often we are thinking about implanting younger children, but we also want to think about the other end of the age spectrum. Over the years there have been at least two questions that keep coming up: will these older people obtain the kind of benefit we see in young children, and is there a greater risk of adverse surgical events in this population?
The first article is the primary one which has to do with cochlear implants (Carlson, Breen, Gifford, Driscoll, Neff, et al., 2010). The authors of this article found that the older recipients, 50 of them over the age of 79 years, actually received benefits that were similar to those obtained by younger patients. In this particular article, they did not believe that the surgical risks were considerable. The older subjects demonstrated no greater risks of adverse surgical events. These subjects did demonstrate greater risk of perioperative anesthetic complications, but the authors state that the anesthesiologists were well aware of this.
These findings tie to two articles by Lin and colleagues ( Lin, Ferrucci, Metter, An, Zonderman, & Resnick, 2011; Lin, Metter, O'Brien, Resnick, Zonderman, & Ferrucci, 2011). Some of you have probably seen the series of articles published recently, showing findings suggesting that presbycusis can be linked to poor cognitive functioning and social isolation. There is research showing that hearing loss may be related to cortical processing. The notion here is that as more and more people are living to be upwards of 90 and 100 years, examining cochlear implant candidacy for people over the age of 80 or so has become a reasonable consideration. They are an interesting series of articles that I encourage you to read.
Speaking of checking things out, I encourage you to check out René's two 20Q articles on AudiologyOnline (Gifford, 2011, May; Gifford, 2011, June) for a great review of the latest research on cochlear implants.
Gus's Pick of the Month
As part of the Vanderbilt Audiology Journal Club sessions, I typically select an article that I have found interesting and clinically relevant. One of the topics I think you are all familiar with, even if you do not routinely fit hearing aids, is the general thought that two hearing aids are better than one. I fact, I imagine many of you have told your patients that two hearing aids are better than one.
There are several reasons for this; one reason relates to improved understanding of speech in background noise. There are two reasons why we believe this should be true. One of them is binaural redundancy, meaning the brain actually gets two looks at the signal of interest. The second is binaural squelch, where the brain can use phase and amplitude differences to help distinguish and differentiate the signal. By using this, the ears can actually improve the signal-to-noise ratio (SNR) at the level of the brainstem. This is, to some extent, related to the masking level difference (MLD) that some of you may have used back in the days when we used to do MLDs diagnostically.
In 2005, a study was published by Therese and Brian Walden entitled Unilateral versus Bilateral Amplification for Adults with Impaired Hearing, and a lot of people were talking about it. These authors showed SNR loss scores (i.e., from the QuickSIN) when individuals were fitted with two hearing aids compared to their right and left ears monaurally. Not only was their performance not better with two hearing aids, it actually was somewhat worse. This got a lot of attention because it was contradictory to everything we knew about using two hearing aids up to that point. It was a topic of conversation at several conferences. There were some possible reasons, however, as to why these results might have occurred.
At these high intensity levels, perhaps the non-test ear was still responding because these people did not have severe hearing losses. It also could be that the hearing aids only had minimal gain. It could be that the hearing aids were in saturation, which would minimize signal-to-noise differences. Some speculated that since this was a retrospective study, maybe there was some type of ordering effect based on what QuickSIN list or hearing aid conduction was used or tested first? It's hard to say.
When things do not look the way we think they should look, it is common to do a replication study. That is what just came out in Journal of the American Academy of Audiology (JAAA) from Rachel McArdle and her colleagues (McArdle, Killion, Mennite, & Chisolm, 2012). They more or less used the same protocol as Walden and Walden (2005). They divided their study groups into better hearing and poorer hearing. They tested in the following conditions: unaided, right ear aided with the left ear plugged, and right ear aided with the left ear open. When the left ear was open, performance improved significantly by about 3 dB or so, which does indicate that if you leave the opposite ear open, you really are not testing monaurally. When using a loud presentation level (i.e., 75 dB HL in this study) you are likely testing bilaterally unless the person has a severe hearing loss in the unaided ear.
The good news, for those of you who believe that two hearing aids indeed are better than one, is that the overall winner was the binaural condition, although it was not terribly better than the best-hearing ear. Remember, however, that this was conducted using the same protocol as Walden and Walden, so that speech and noise were coming from the same loudspeaker. McArdle et al. (2012) then went a step further in this study, and they recorded restaurant noise from eight speakers surrounding the KEMAR. After making the recording, they played it through earphones to subjects so the subjects could listen to the right ear or left ear only, either the right or left ear recording presented to both ears (a diotic presentation) or the individual signals recorded from each ear presented to each ear—a true binaural presentation.
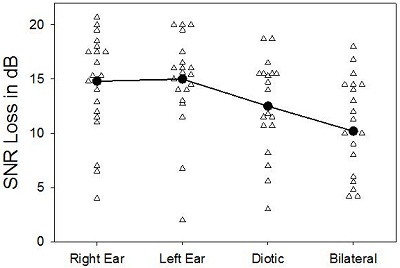
Figure 1. Results of SNR loss testing in four conditions: Right ear, left ear, diotic, and bilateral, adapted from McArdle et al., 2012. The solid line represents the average of each condition.
The results of this testing is shown in Figure 1 (adapted from McArdle et al., 2012). There was about a 2 dB benefit for the diotic presentation over the unilateral, which would reflect the redundancy advantage. The signals were also presented bilaterally, where the subjects could pick up the spatial differences. There was another 2 dB benefit. So we now have a 4 dB benefit using two hearing aids versus one. I am glad to report that the world is back in the way it was supposed to be from graduate school. Indeed, it does appear again that two hearing aids are better than one, at least at this point.
That takes us to our keynote speaker for today, Dr. McCaslin. Dr. McCaslin was a guest at the journal club about a year ago, but a lot has happened with Devin professionally in the past year. He was promoted to Associate Professor at Vandy. He was elected to the Academy's Board of Directors. Thank you, Devin for volunteering for that job. He has also been named the Deputy Editor of the Journal of the American Academy of Audiology. So with that, I am going to turn things over to our keynote speaker, Dr. McCaslin.
Devin McCaslin: Thank you, Gus. Last year in the Vanderbilt Audiology Journal Club I talked about the ocular cervical vestibular-evoked myogenic potential (oVEMP). That is one of the most exciting things happening in vestibular function testing right now. But a lot has happened with the head impulse test since I spoke, and I think it is going to change how we evaluate and treat patients with vestibular disorders.
I will go through two articles that show how the video head impulse test was created for diagnostic purposes. Then I will show you an article that is actually an ePub, so it is ahead of publication. It is a wonderful review article with some speculation into what the video head impulse can tell us about rehabilitation of vestibular patients and how central nervous system compensation may not be exactly what we thought it was. By having this test, it will change the way we look at things and, I think, the way we do things.
I want to start first with some background on the head impulse test, both where it came from and its historical underpinnings. Then we will cover the articles. The head impulse test is really a measure of the vestibulo-ocular reflex (VOR). This is a reflex that acts to keep the eyes stable when we move our head. If you move your head 45 degrees to the left, the eyes will be driven 45 degrees to the right. This makes it possible to run on a treadmill; when you move up and down, your eyes go up and down. When you go up, your eye goes down. If you go down, your eye goes up. Furthermore, you can successfully read a magazine while you are running or moving. This is mediated by a reflex. I am not going to go through all of it today, but it is driven by the peripheral vestibular system. The horizontal VOR runs into the vestibular nuclear complex, the abducens and the ocular motor nucleus.
Through its connections to the extraocular eye muscles, we get a slow deviation of the eye in the opposite direction of the head movement. That is generated by the vestibular system. That is why, when we do caloric tests or rotational testing, we measure the slow phase velocity. The response is being generated by the vestibular system. Then the ocular motor system comes in and turns off the vestibular system very briefly and resets the eye in the orbit so it can start over again. That is what we see as nystagmus and generates what we know as a VOR.
Journal Articles
Over two decades years ago Halmagyi and Curthoys (1988) reported that the head impulse test could be used as an evaluation of vestibular system function. The head impulse test is a screening test. It uses what is called the doll's eye reflex, or the oculocephalic reflex. This was originally used on patients in a coma. Someone would move the patient's head, and if the eyes moved in the opposite direction of the head movement they knew that the patient had some brainstem function. This could be used as a prognosticator for perhaps how well the patient was going to recover. Halmagyi and Curthoys (1988) noted that when you move a head towards the impaired side, you would see a catch-up saccade in the direction of the impaired system after the head is moved.
The head impulse test has technically been qualified as a bedside test up to this time. The video head impulse test system will let us become very quantitative in our measurements. Before this, the tester only observed the eye movements, not quantitative measurements. To perform the test, the examiner gently grasps the patient's head and passively guides it through a quick head turn. These are very low amplitude, high velocity head movements, almost like a jerk. Before the head is turned, the patient is instructed to fixate on a target and maintain focus on it throughout the head turn. You say, "I am going to move your head very quickly, but your job is to keep your eyes on the target the entire time."
We usually tilt the patient's head forward 30 degrees so that we bring the lateral canals into the plane of stimulation. The horizontal semicircular canals are angled upwards 30 degrees when we are standing upright. Usually when you walk, your head is tilted downward. You have it tilted down about 30 degrees when you are scanning. To perform the head thrust, gently grasp the patient's head on both sides. Rapidly and abruptly, move the patient's head 15 to 20 degrees to one side. Typically we do this in an unpredictable manner.
What we are looking for is a catch-up saccade. This would suggest that the VOR is not driving the eye 180 degrees out of phase. If I move my head 45 degrees to the left, my eyes may have only gone 25 degrees to the right. So what happened is the eye traveled with the head in the same direction as the head for a certain number of degrees, and when the head stops the eye is no longer on target. The eye then has to go and reacquire the target. This reacquisition of the target is known as the catch-up saccade, and that is what the tester is looking for. That would be an abnormal response.
The big question was always, "How sensitive is this head impulse test?" The literature asked how much of a caloric weakness do you have to have in order for the head impulse test to become positive? What most literature shows is around 42.5 percent (Perez & Rama-Lopez, 2003). Where you start seeing catch-up saccades occur in the head impulse test is where there is a caloric weakness around 40 percent. I would say that is probably pretty consistent to what we are seeing even today.
That was then. We did not have a sophisticated video system with high sampling rates that were able to track and sample the saccade. When you take a movie, you get little pictures, and you put them together and that is the frame rate. The frame rates were too slow up until recently to record the saccade accurately because it happens so quickly. Now the technology is advanced. What we thought we knew now is completely different. It is almost like we used to have Galileo looking with a telescope, and now we have the Hubble telescope. With the video head impulse test and these new cameras, a whole new body of literature is starting to emerge about compensation and how the vestibular system heals itself after it is impaired.
The first articles I am going to talk about are two seminal articles that came out in 2009. There was the big push to try to develop the video head impulse test, and have a system that would be light enough so it would not move on the head. You have to move the head very quickly. You have to jerk it 2,000 degrees per second squared. The goggles would move because they are fairly large, and the camera is not going to be able to track the eye. We are trying to be very sensitive and track the pupil. So the race was on to try to develop a lightweight set of goggles that had all that technology packed into them. In 2009, a couple of these systems came out. I will present the work from each of them here.
The first, out of Germany, is from Bartl, Lehnen, Kohlbecher and Schneider (2009). They published a report of a high-frame rate video system that would give an accurate measure of the VOR gain when the head was moved. It was equivalent to a search coil. So, you ask, what is a search coil? A search coil is a coil that is embedded into what almost looks like a contact lens (Figure 2). This was what we always used to use before we had access to these video systems. When we wanted very high resolution of the VOR measured, we would use this system. It is like a rubber ring and it adheres right to the eye. It creates a magnetic field.
Figure 2. Scleral search coil on the eye.
There are magnets around the eye that are all producing a different frequency. When you move the head, the magnets pick up the movement of the eye. Usually these multiple magnets are oriented orthogonally around the eye, and you get a very good measurement with less than one degree of movement with great temporal resolution. You get everything you need to track how the VOR is working. But as you can imagine, it is not a fun thing to do. You have to stick this big contact lens in somebody's eye in order to get a good measure. That is why video was so appealing because it only required a pair of goggles. You did not have to stick anything in the patient's eye. The point of developing these systems was to maintain sensitivity and specificity of the scleral search coil, which is very noxious, but in a system that is easy to use and lightweight.
Figure 3 shows one of the first systems, called the EyeSeeCam. It happens to be the one that we use. It looks like a very small set of racquetball goggles or swim goggles with the same sort of system that we use in our video recordings for calorics or ocular motor testing. We have the dichroic mirrors, which are mirrors coated with a metallic film that allows reflection of the eye back to the camera but also allows the person being tested to look through them. It was packing all of these cameras and the glass into a system that would not move when the head moved.

Figure 3. EyeSeeCam.
When you collect the video head-impulse-test responses, the system tracks the pupil of the eye, just as in videonystagmography (VNG) recordings that we are used to. It gives a display of the head movement and the eye movement. Because of the VOR, when you move your head 45 degrees to the left, your eyes should move 45 degrees to the right. With the head movement you get an equal and opposite corresponding eye movement. The measurement Bartl et al. (2009) used was gain. Gain is described in velocity, as the eye velocity divided by the head velocity. Those values should be equal if the VOR is functioning properly.
Figure 4 is an example of a normal patient we tested using the system they published on. The blue tracing is eye velocity and the red is the head velocity. Velocity is measured on the y-axis and time on the x-axis. You see that the head movement occurred over almost 100 milliseconds. The eye velocity when you move the head is almost the exact representation of what the head movement was. If you were to overlay those two tracings, you would not even be able to tell the difference. This is one of the first reports indicating that we can use video to do this.
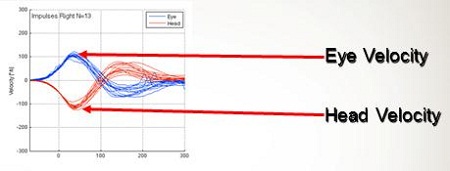
Figure 4. Head impulse test measurement of a normal subject as velocity over time. Blue tracings are the eye velocity and red tracings are head velocity.
Using the video system, we can see almost the same things as the scleral search coil. The search coil is the gold standard in that condition (Robinson, 1963). This is what we have used before. When using goggles with this type of test, the goggles still slip enough to cause artifact in the tracing. That has since been fixed in a lot of these systems. This is proof that, yes, you can develop a system that will track the VOR with the same temporal and spacial resolution as a scleral search coil.
Figure 5 shows what a complete tracing looks like, with both head impulses to the right and left. You get a gain measure dividing eye by head velocity. Usually that is going to be about one. The purple and blue dot plot shows a gain of very close to one, meaning that the eye movement and head movement were equivalent. You can also display these in a three-dimensional format (Figure 5). The salmon color below tracing is the eye movement and the light green tracing is the head movement. The eye and the head movements are almost exactly the same.

Figure 5. 3D view of head impulse tests results. Salmon is eye movement and green is head movement.
Vandy Vignette - Lewis Nashner
Gus Mueller: In each Vanderbilt Journal Club seminar, we take a quick break about halfway through the journal articles to share an audiology vignette from Vanderbilt University. Dr. McCaslin suggested today's vignette about an adjunct professor at Vanderbilt, in our Vestibular Sciences program, who invented platform posturography. His name is Lewis Nashner, and he invented platform posturogrpahy when he was at MIT. There you have it.
Journal Review Continued
Devin McCaslin: The next article is also out of Australia and was published about the same time as the Bartl et al study (2009). This one is by MacDougall, Weber, McGarvie, Halmagyi and Curthoys (2009). The last two authors are the same people that described the bedside test almost 24 years ago. This study done was to determine if the diagnostic accuracy of the video head impulse test, again, was comparable to scleral search coils. It was also to define what abnormal gain measurements would be, and also to discuss the different types of saccades. The video head impulse test has now led us to discover that there are actually two different types of saccades.
First is an overt saccade, which is the one that we see during bedside testing when the head is moved and the eye jumps back to reacquire the target. But there are also covert saccades. They are probably one of the most interesting things we are going to find in the next few years. Covert saccades were not visible when we were moving the head because they occur during the head movement. You could not see them when you were moving the head very quickly. Now with the video system, however, we can see them.
As I said previously, the search coil method was the gold standard (Robinson, 1963). But this group (MacDougall et el., 2009) wanted to take a group of healthy people and a group of people with various vestibular system impairments and compare them. Essentially, they wanted to discuss the sensitivity and specificity of the video impulse test. They took 16 subjects and used both video head impulse testing and search coils simultaneously. That was a great way to do it. They took eight neurologically intact adults for the control group and they took six patients with vestibular neuritis for the experimental group. One patient had Meniere's disease and another patient had confirmed bilateral vestibular-system impairment. The subjects were to fixate on a laser dot on the wall as the target. They performed 50 head impulses to each side in an unpredictable manner. So, they randomized the direction. The same eye that was recorded during the video head impulse test was also recorded during the scleral search coil test. They found that a gain of less than .68 in this study considered abnormal gain. They did a paired sample T-test, which you can do when you are using the same subjects within the groups.
So, how well did one predict the other? Numerically, a perfect correlation would be 1.0. So, the closer you are to 1.0, the better the correlation is. The authors found that head movement acceleration between video head impulse and search coils were highly correlated at .999. It does not get any better than that. Eye movement acceleration between the video head impulse test and search coils was also highly correlated at .93. The gain of the video head impulse test and search coils was not significantly different. This study confirmed that you can use this video system to gain the same quality of information about the vestibular sytem as you could with the search coils.
Why is this important? Now we have a measure. You know that if a person has a gain of .68 or less they most likely have a vestibular system impairment on the side that is being thrust. And you should feel pretty confident, as confident as you did with scleral search coils because the sensitivity and specificity of it, in these cases, was one for identifying the abnormals versus normal. Does this matter clinically? Yes, it does. These findings would suggest that the head impulse test is equivalent to search coils in identifying peripheral vestibular end-organ impairments.
Results need to be examined in various pathologies and severities of impairment. If there is a testing system that is sensitive to impairments, we really need to look at identifying different impairments. There are several vestibular disorders arising from different sorts of pathologies. It will be interesting to see what research unfolds as a result of this technology.
Figure 6 is an example of patient with a left unilateral weakness of 54 percent by caloric testing. You can see the head movement (red) and the eye movement (blue) in relation to each other. The right side was normal. On the left side you can see the catch-up saccades on the blue eye movement, indicated by the black arrow. The gain was actually relatively normal, but the catch-up saccades are in the direction of the intact side. What happened was the eye was trying to reacquire that target. That is what you can see on the video recording.
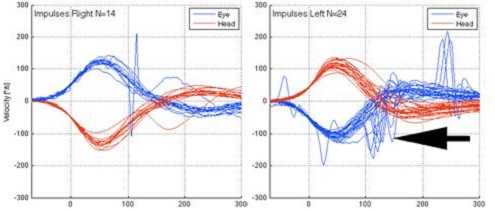
Figure 6. Head impulse test on patient with left unilateral weakness. Catch-up saccades are noted in the eye movement on the left side by the black arrow.
Even after this study, however, there is no system that establishes what it is abnormal or normal. I think most people are approaching this like reading an ABR. You have to find the presence of the saccades. The interpretation is up to the audiologist. Some systems are a little more advanced than others. Some have algorithms that run the responses and determine whether they are valid or not. Figure 7 shows the three-dimensional version of the unilateral weakness. The overt saccades are the spikes, where the eye is jumping back to reacquire the target.

Figure 7. Head impulse test in 3-D view, with spikes indicating overt saccades.
Figure 8 is a tracing from a patient with a bilateral caloric weakness. The catch-up saccades are noted both to the right and to the left.
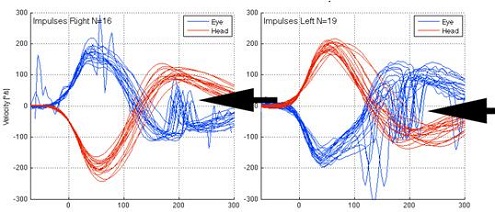
Figure 8. Head impulse test from patient with bilateral caloric weakness. The overt saccases are noted on both right and left sides by black arrows.
Commodore Award
Gus Mueller: During each Vanderbilt Audiology Journal Club session, we designate one article as receiving the Commodore Award, named of course after Commodore Cornelius Vanderbilt, whose generous gift in 1873 resulted in the founding of Vanderbilt University. In the opinion of our presenter, the article receiving the Commodore Award has particular importance, significance, relevance or is unique is some way.
Devin McCaslin: For the Commodore Award today, I have selected an article entitled Plasticity During Vestibular Compensation: The Role of Saccades (MacDougall & Curthoys, 2012). I think most people have taken the head impulse test from a diagnostic point of view. The question to be answered was if we could diagnose a vestibular system impairment using this system relatively easily. What this study shows is that saccades can teach us more about what is happening with vestibular system compensation. Vestibular compensation may not be mediated exactly as we thought it was.
These authors reviewed the two concepts of static and dynamic compensation. When you lose an end organ, you are going to have static compensation, which is generally defined as spontaneous nystagmus. Once spontaneous nystagmus disappears we say that the system is static or that the person is statically compensated. There is also another type of compensation when you lose an end organ, dynamic compensation. This happens in people who do not have any more spontaneous nystagmus, but still complain of head movements. "I do not like when I move my head quickly because everything blurs and it makes me feel a little bit strange. I am still a little bit unsteady." Even though the VOR has been statically compensated, there are still dynamic issues that patients will complain about.
Why does learning about compensation matter? According to this group, there are about 20 to 30 percent of patients with unilateral vestibular-system impairments that do not compensate well. You see these patients in your clinics. They go through vestibular rehabilitation, and they still do not feel great. Something I have noticed as we have started a pediatric vestibular clinic is that children who have almost minimal or no vestibular system function do very well. They are not slowed down at all. If you saw this in an adult, they would come in to you in a wheelchair. But I have seen these younger children, some of them with cochlear implants and no vestibular system function, test just fine. They run around and play various sports. There may be some reasons to explain that, as I will demonstrate in this article. By using this video head impulse technology, it appears that compensation in adults does not occur exactly as we thought it did before.
The idea of this review is that very early studies of compensation suggested there was considerable recovery of the dynamic VOR. What has now been discovered is that when you lose enough function to affect the high frequencies of the VOR, there is almost no compensation or replacement for those high frequencies. It happens in the low frequencies but does not seem to help the high frequencies. So even if you have a rotational testing system, those responses are still considered relatively low frequency. You are looking at tens of degrees per second of a rotation versus the head impulse test, which is thousands of degrees per second. So what you see in a person that loses an end organ on one side is their phase and gain and rotational testing will go back to normal. So we say, "Well, you should be feeling great because you have now dynamically compensated."
The VOR is doing exactly what it should in terms of timing and gain. But this new system has shown us that when you lose function in the high frequencies, it is not replaced by the vestibular system the same as it is in the low frequencies. There is a different mechanism being used. The authors suggest that there are distinctly different systems for compensating low and high-frequency impairments. Since the majority of patients with high-frequency impairments recover well from a unilateral vestibular deficiency, the authors suggested this was due to two primary concepts. The small recovery and low-frequency function may be enough to make them feel better, or they are using other strategies to overcome the loss of high-frequency impairment. This is what the high frame-rate technology has allowed us to see. It appears that when you move your head at a high frequency, the vestibular system is not replaced. In fact, something else happens. This group suggests that the ocular motor system is replacing what is happening in the vestibular system. So if a patient with a unilateral impairment is asked to keep their gaze fixed on a target while the head is moved, they can learn to do so. They can learn to modulate their saccades that occur during the head movement.
After the head is done moving during the impulse test, the brain generates an overt saccade to reacquire the target. But there are no saccades occurring during the head movement. As the person compensates, the saccades actually get earlier and earlier in time. They actually occur during the head movement. Now what happens is the person has the impairment on one side, and the ocular motor system is generating a saccade during that very brief head movement, not the vestibular system. Because that saccade has been generated in the opposite direction of the head movement, when the head stops, the eye is not as far off the target as it would have been, so that helps in compensation. It senses the head moving and generates a saccade in the opposite direction. When the head stops, the eye is closer to the target then it normally would have been.
Figure 9 is an example of a patient that has a bilateral weakness. During the head movement, there are saccades being generated that are almost instantaneous. The red line is the head movement and the blue is the eye movement. During the head impulse movement, you can see the saccades that are being generated during that head movement.
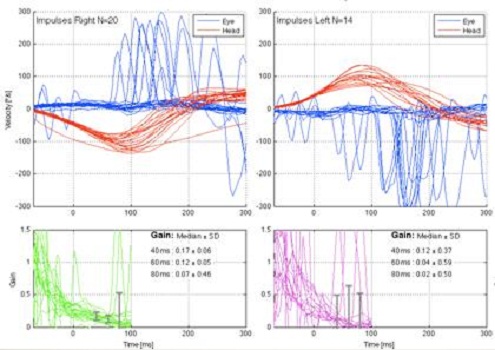
Figure 9. Patient with bilateral vestibular weakness. Saccades are noted on the blue tracings for each side.
MacDougall and Curthoys (2012) reported one case of a child who was given gentamicin or a vestibulotoxic antibiotic at an early age and lost all vestibular function. Today, this same child runs around and is doing fine. They found that the ocular motor system is replacing what the vestibular system was doing. If you think about it, this can change the way we approach rehabilitation. Now it looks like the ocular motor system is doing the heavy lifting for normal head movements, not the cerebellum and the vestibular nuclei. It is actually generating a saccade rather than using the vestibular system. That will probably change the way that many people approach rehabilitation. They may be trained to generate ocular saccades.
I think back, again, to the pediatric vestibular clinic that we have. I think that when you are young, you must be able to learn this very easily early on. All the senses and the associated brain function is more plastic in children. It is baffling to me to see children with no measureable vestibular system function, yet they are walking around and playing. One child reported to me that sometimes he would have to stop a little longer than others to reorient on the ball when playing soccer, but other than that he was fine.
I am sure in the next couple of months you will see more data on what we thought we were doing in vestibular rehabilitation and how what is actually going on is much different now due to frame-rate sampling. Not only is the video head impulse test going to be great for diagnostic purposes, but it is likely going to give us some insight into what is happening physiologically and change what we do with rehabilitation.
Bartl, K., Lehnen, N., Kohlbecher, S., & Schneider, E. (2009). Head impulse testing using video-oculography. Anals of the New York Academy of Sciences, 1164, 331-333.
Carlson, M.L., Breen, J.T., Gifford, R.H., Driscoll, C.L., Neff, B.A., Beatty, C.W., Peterson, A.M., & Olund, A.P. (2010). Cochlear implantation in the octogenarian and nonagenarian. Otology & Neurotology, 31(8), 1343-1349.
Gifford, R. (2011, May 2). 20Q: hearing preservation with cochlear implantation. AudiologyOnline, Article 2363. Direct URL: www.audiologyonline.com/articles/article_detail.asp?article_id=2363. Retrieved July 9, 2012, from the Articles Archive on www.AudiologyOnline.com.
Gifford, R. (2011, June 6). 20Q: hybrid/EAS cochlear implants - new research and clinical tips. AudiologyOnline, Article 2367. Direct URL: www.audiologyonline.com/articles/article_detail.asp?article_id=2367. Retrieved July 9, 2012, from the Articles Archive on www.AudiologyOnline.com.
Halmagyi, G.M., & Curthoys, I.S. (1988). A clinical sign of canal paresis. Archives of Neurology, 45(7), 737-739.
Lin, F.R., Ferrucci, L., Metter, E.J., An, Y., Zonderman, A.B., & Resnick, S.M. (2011). Hearing loss and cognition in the Baltimore longitudinal study of aging. Neuropsychology, 25(6), 763-770.
Lin, F.R., Metter, E.J., O'Brien, R.J., Resnick, S.M., Zonderman, A.B., & Ferrucci, L. (2011). Hearing loss and incident dementia. Archives of Neurology, 68(2), 214-220.
MacDougall, H.G., & Curthoys, I.S. (2012). Plasticity during vestibular compensation: the role of saccades. Frontiers in Neurology, 3(21), Epub.
MacDougall, H.G., Weber, K.P., McGarvie, L.A., Halmagyi, G.M., & Curthoys, I.S. (2009). The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology, 73(14), 1134-1141.
McArdle, R.A., Killion, M., Mennite, M.A., & Chisolm, T.H. (2012). Are two ears not better than one? Journal of the American Academy of Audiology, 23(3), 171-181.
Perez, N., & Rama-Lopez, J. (2003). Head-impulse and caloric tests in patients with dizziness. Otology & Neurotology, 24(6), 913-917.
Robinson, D.A. (1963). A method of measuring eye movement using a scleral search coil in a magnetic field. Bio-medical electronics, 10(4), 137-145.
Walden, T.C., & Walden, B.E. (2005). Unilateral versus bilateral amplification for adults with impaired hearing. Journal of the American Academy of Audiology, 16(8), 574-584.