Learning Outcomes
After this course learners will be able to:
- Describe the characteristics of patients who fail to perceive motion following caloric irrigation.
- Describe the difference between the vHIT and the SHIMP.
- Describe the best vibration frequency and location to place the stimulator.
Absence of Rotation Perception During Warm Water Caloric Irrigation in Some Seniors with Postural Instability (Chiarovano et al., 2016)
Dr. Gary Jacobson: The paper I'm reviewing was authored by Chiarovano and colleagues (Chiarovano et al., 2016) and appeared in the journal Frontiers of Neurology. In the introduction, the authors state that it's impossible for about 2/3 of individuals over the age of 70 years to stand on a foam surface with their eyes closed for 30 seconds. Further, 1/3 of individuals over the age of 65 years fall at least one time each year. This finding implies that these individuals may be at risk for falls, especially in darkness. The authors report that the otolith end organs are more affected by age than are the semi-circular canals. They report that aging increases the variability in the threshold of perception of motion. Lastly, there is evidence that age reduces the activation of the vestibular cortex following caloric stimulation.
Purpose
Investigators noted that to date, no one has examined the effect of age on the perception of movement following caloric stimulation. Furthermore, the relationship between the absence of motion perception following caloric stimulation and falls also had not been examined. Therefore, the investigators examined two groups of subjects. One group had postural instability and failed to perceive motion during caloric testing. A second group was comprised of age-matched normal controls. The authors hypothesized that falls in the elderly may be caused by a disorder affecting central processing of normally-coded peripheral inputs. The article states that "falls in the elderly may result from a 'vestibular neglect' due to an inappropriate central processing of normal vestibular peripheral inputs." Note that the term "vestibular neglect" is the authors' term.
Methods
There were 20 subjects in this study. There were 10 elders with a mean age of 77 years who complained of postural instability (i.e., they complained that they felt like they were rocking on a boat). The control group was 10 sex-matched and age-matched elders without complaints of postural instability. The mean age of the control group was 74 years. All subjects underwent bithermal caloric testing. Normal bithermal caloric asymmetry was no greater than 25%, although they used data generated by the warm irrigations only for the study. Both warm caloric irrigations had to exceed 15 degrees per second in mean peak slow phase eye velocity in order for the individuals to participate in the study. Also, subjects in the abnormal group failed to report a sensation of rotation following warm caloric irrigations.
Measures
The measures that were treated as dependent variables were a Dizziness Handicap Inventory (DHI) total score, Video Head Impulse Test (vHIT) gain, VEMP amplitudes, latencies, and equilibrium scores on the SOT of the equitest protocol. In addition to a bithermal caloric test, subjects completed the DHI, horizontal canal vHIT testing, both cVEMP and oVEMP tests to both air and bone conducted stimuli, Equitest computerized dynamic posturography, tests for head-shake nystagmus, test for vibration-induced nystagmus, and audiometric testing.
Results
DHI. The mean total DHI score for the abnormal group was 39 out of 100 points, representing on average, moderate self-report dizziness handicap. The mean total DHI score for the control group was 14 out of 100 points, representing an absence of self-report dizziness handicap. Not surprisingly, these differences were statistically significant. The unsteady subjects who failed to perceive motion during caloric testing self-reported a significantly larger effect of that disorder on their dizziness disability handicap.
Caloric Test. This result showed no significant group differences in caloric maximum peak slow phase velocity for warm or cool water irrigations. All patients failed to report a sensation of rotation after warm caloric irrigations. All control subjects did report a sensation of head rotation, or head and body rotation, during caloric testing.
vHIT. There were no significant group differences in the mean horizontal vestibulo-ocular reflex gain derived from vHIT testing.
cVEMP. There were no significant group differences in cVEMP latencies or amplitudes in response to air-conducted tone bursts when the control and patient groups were compared. It is noteworthy that 40% of the patients and 30% of the controls failed to generate cVEMPs to air-conducted stimuli.
oVEMP. oVEMP testing to air-conducted tone bursts showed significantly reduced amplitudes for the patient group. The finding of a unilaterally-reduced amplitude in a patient would argue for an impairment affecting either or both the utricle or superior vestibular nerve. If we add to that a normal caloric test, that would argue for a utricular impairment in isolation. It has been reported that patients with unilateral utricular impairments do not complain of vertigo, but instead experience postural instability and a sensation that they “feel as though they might fall.” There were no significant differences in latency. It is noteworthy, that 60% of patients and 30% of controls failed to generate an oVEMP. However, in response to the same tone bursts delivered by bone conduction at the mastoid, there were no significant group differences in oVEMP amplitudes or latencies. In contrast to the air-conducted tone burst, only 20% of patients and 10% of controls failed to generate an oVEMP to the vibratory stimulus. This finding suggests that the absent responses to air-conducted stimuli occurred because they could not produce an air-conducted stimulus with sufficient intensity to translate the otolith mass in both patient and control groups.
Equitest. For CDP, the control group showed normal performance. However, the patient group failed to maintain postural stability in both condition five (eyes closed, sway referenced platform) and condition six (eyes open, sway referenced platform and vision). In the patient group, 80% of participants fell under conditions five and six. The remaining 20% of participants scored abnormally low on condition five and fell on condition six. Condition five is the condition most sensitive to impairments affecting both the somesthetic and peripheral vestibular systems.
Summary
Unlike the control subjects, patients were unsteady, generated a normal caloric response, did not perceive motion in the post-irrigation interval, and generated significantly smaller oVEMPs in response to acoustical stimulation (as opposed to vibration stimulation).
Discussion
The patient group failed to perceive rotation following warm caloric irrigation, even when the mean peak slow phase velocities exceeded 15 degrees per second. This was part of the subject selection criteria. The absence of motion perception was associated with an inadequate postural strategy to maintain balance on conditions five and six. Further, "absence of perception of movement during the caloric test may be a marker for risk of falls, which has not been considered before." Investigators have suggested that this phenomenon of a normal caloric response and absent perception of self-rotation in an unsteady patient may be considered a form of "vestibular neglect", which suggests a central vestibular system impairment.
Investigators have summarized their belief that a relationship exists between a deficit of central processing (which they refer to as "vestibular neglect") and postural instability. Additionally, the investigators suggest that the lack of self-motion perception during the caloric test in unsteady patients should draw the clinician's attention to the need to implement measures to prevent falls.
The investigators ended their paper with a series of speculations of how it's possible to have a normal horizontal VOR and abnormal postural stability. For example, the vestibulo-ocular reflex is a simple three-neuron arc. In contrast, the circuitry underlying perception of self-motion is polysynaptic and a distributed phenomenon in the brain. This means that the pathway of motion perception, since it is distributed throughout the brain, may be more sensitive to the effects of age and disease than a simple reflex arc.
Shortcomings
We identified a number of shortcomings in this study. First, the criteria used to select subjects increased the likelihood there would be differences in semi-objective measures of postural instability. Second, there was no attempt to measure the integrity of the lower extremity somatosenses. The somatosenses provide essential information to maintain static standing balance. In comparison, the vestibulospinal system is activated by head movement, and movement of the head and body together. Third, the group differences in stimulus level required to generate the oVEMP suggested that there were significant differences between groups in the functional integrity of the utriculo-ocular reflex. In the clinic, patients who show absent oVEMPs in response to air-conducted tone bursts complain primarily of postural instability, as did the patients in the current report. We question whether utricular impairments played a role in the patient's failure to experience post-caloric vertigo. Lastly, while the horizontal vHIT data provided useful information about the horizontal semi-circular canals, investigators did not asses the functional status of the vertical canals, which may have contributed to the patients' unsteadiness but would not explain the patients' failure to perceive vertigo during caloric testing.
Conclusion
In conclusion, the observed dissociation between motion perception during caloric testing and post-caloric nystagmus may be a determinant of postural instability, as it was these same seniors who demonstrated greater postural instability compared to age-matched controls. Therefore, failure to perceive rotation during caloric testing when the slow phase velocity is greater than 15 degrees per second should encourage the clinician to consider preventive actions to mitigate falls risk, such as vestibular rehabilitative therapy. Further studies are needed to evaluate the proportion of seniors with and without motion perception during caloric testing amongst a larger population of patients with and without complaints of postural instability.
Age Predicts the Absence of Caloric-Induced Vertigo (Jacobson, Piker, Grantham, & English, 2017)
Next, I will briefly mention a study that we conducted which is now in press. We conducted the investigation over a three-month period. We were interested in knowing whether the lack of vertigo perception in the post-warm caloric irrigation interval occurred only in the elderly. Our findings were collected from a total of 92 subjects. All subjects were patients of our clinic and completed comprehensive vestibular function studies. The subjects were classified as normal or abnormal based on their response to the question, "Other than the temperature of the water, did you have any other sensation after the water stopped flowing in your ear?". Those who reported a perception of motion in the post-irrigation interval were placed in the "with sensation" group. Subjects who failed to perceive motion after the caloric irrigation were placed in the "without sensation" group. Parallel with the previous study, subjects were required to generate a nystagmus that had a mean peak slow phase velocity of greater than 15 degrees per second. We were interested in knowing whether the mean ages of the subjects in the two groups were significantly different. Our hypothesis was that the mean age in those subjects without perception of motion would be significantly older than those with perception of motion.
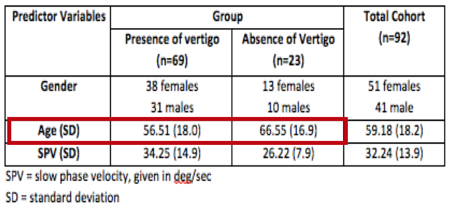
Results showed that both age and mean peak slow phase velocity of the caloric response were significant predictors of vertigo perception during the caloric exam. Increased age was associated with increased caloric SPV and decreased perception of motion. The association between the age and caloric perception was not specific to the older patients. Half of the patients reporting an absence of vertigo were under the age of 65 years.
Table 1 shows that the mean age of the group with perception of motion was approximately 10 years younger than the mean age of the group without sensation of motion. Specifically, the mean age of subjects with perception of motion was 56 years, and the mean age of the subjects without perception of rotation was 66 years. These differences were statistically significant. By our measures, the lack of post-irrigation motion perception did not occur exclusively for elderly subjects. It is our opinion that additional research is justified (e.g., evaluating the relationship, if any, between periventricular white matter disease and lack of post-caloric vertigo). All of these studies have rekindled an interest in the clinical assessment of motion perception.
Table 1. Demographics of both groups.
How to Perform the Skull Vibration-Induced Nystagmus Test (SVINT) (Dumas, Perrin, Ouedraogo, & Schmerber, 2016)
Dr. Richard Roberts: The skull vibration-induced nystagmus test (SVINT) is an example of existing technology used in other ways to generate or create more information that's useful in the clinic. This is especially important when we're dealing with decreased reimbursement and expensive equipment. In an article by Dumas and colleagues, they discuss the practical methods to complete this test, as well as the key findings the clinician could expect to observe during the test (Dumas et al., 2016). Another name they use to refer to the SVINT is “The Vestibular Weber”, in comparison to the Weber test that we learned about in our "Introduction to Audiology" days. The presence of nystagmus generated by the vestibular system can create a vestibular asymmetry. This is usually related to a peripheral vestibular asymmetry. Using this low-tech procedure, we can quickly determine whether asymmetry of vestibular function exists, as well as the side of the vestibular loss.
Purpose
The basis for the SVINT test has to do with the fact that if we apply vibration to the skull, we can induce sufficient labyrinthine fluid displacement to activate Type I hair cells, not only in the semicircular canals but also in the otolith organs, to create measurable, phase-locked afferent firing. Ian Curthoys has done a lot of work in this area to prove that we truly are getting the stimulation to all of the vestibular structures simultaneously. He has measured phase-locked neural firing from irregular afferent fibers that synapse with the Type I hair cells, not only in the otolith structures but specifically in the horizontal and anterior semicircular canals in the guinea pig model.
Another interesting observation is that the preferred vibratory stimulus is 100 Hz. If we use that particular frequency, and there's not a lot of work on what frequencies might be best, there is activation of the entire vestibular labyrinth of both ears simultaneously. If we think back to our knowledge of physiology with the vestibular system, when we stimulate both of the ears simultaneously, we are going to see an increase in neural firing from both structures that will arrive at the vestibular nuclei. If we have one side or one structure that is impaired, you're not going to get that same level of neural firing. That will create the asymmetry of the vestibular nuclei, which then is going to create the nystagmus that would result.
Let's step back from vestibular for a second and think about the usefulness of the audiometric Weber test. When we place that tuning fork on the forehead, and we get lateralization of a sound image to one ear, from just that piece of information we know that there is an asymmetric problem. We don't know if we're dealing with a conductive loss (where the image is moving to the ear with the conductive loss), or with a bilateral asymmetric sensorineural hearing loss (where the image is moving to the ear with the less degree of sensorineural hearing loss). This tells us that there's a problem. That's one of the most important things to understand about this test.
Technique
In our clinic, we perform the SVINT during the VNG. Using a 100 Hz vibration for a few seconds, the vibration is applied to each mastoid area and to the vertex. This is done in a vision-denied condition. Since this is usually a peripheral-based nystagmus, if we allow the patient to visually fixate, the vibration would be suppressed by the central ocular motor structure of those pathways. Dumas recommends presenting the vibration for 5 to 10 seconds. My preference is to record for a few seconds to get a baseline and then apply the vibration. You should see that you have a clear onset of the vibration-induced nystagmus that is synced with the application of the vibration, then it stops. The nystagmus would stop as soon as the vibration is turned off. I do the same thing with head shake testing. I like to see that I have a baseline with no nystagmus, and then I generate the nystagmus with that stimulus. There is some suggestion that for Superior Canal Dehiscence, the vertex placement may be a little bit better for most all other issues that are labyrinthine-based. You're going to get a better response with mastoid placement.
What SVINT Tells Us
What does this test tell us? When we're looking at the SVINT, and as we mentioned with the Weber tuning fork test, a lateralized Weber could be related to better hearing ear with unilateral or asymmetric sensorineural hearing loss, or an ear with a conductive hearing loss. The provoked nystagmus gives you an indication that there is an asymmetry, although in this case, we don't really know where the problem is coming from. The SVINT is not very sensitive to central nervous system issues, particularly for brainstem lesions. The sensitivity for CNS issues is around 10 to 30%, which is not that good. When we do provoke nystagmus, again, it's most likely going to be related to a peripheral vestibular issue. You've got a problem in one of the ears.
What SVINT Does Not Tell Us
If a patient has a compensated pattern of dysfunction vs. an uncompensated pattern, the SVINT test would not be able to tell us this. There is evidence that even in patients with a well-compensated vestibular impairment, you can induce nystagmus with vibration. This may relate to the fact that you're stimulating all of the vestibular structures simultaneously, which would not be something that would happen in the natural world. Dumas reports that vibration-induced nystagmus is present whether or not the patient is compensated or uncompensated. You would still want to use other measures during testing to determine that status (e.g., vHIT, head shake nystagmus, rotary chair testing, postural stability, dynamic visual acuity). That nystagmus also does not tell us what's causing the problem, or the specific degree of impairment. You could be looking at an asymmetry that's related to a labyrinthine ischemia, vestibular neuritis, Meniere's disease, Superior Canal Dehiscence.
As far as the degree of impairment, this test is similar to other tests that we collectively refer to as the "bedside tests". Once you get to a caloric asymmetry of 50%, you start to increase the sensitivity of the test. The greater the impairment, the more sensitive the test is. In the end, when you see vibration-induced nystagmus, you could have an asymmetry on the caloric testing from 50% to 100%. If you only have the nystagmus from this test, you didn't have any history, symptoms, or other test results, it would be much more difficult to figure out what's happening with the patient.
SVINT Interpretation
Because it's usually peripheral, we're typically going to have the nystagmus fast phase directed towards the healthy ear. We need to consider just for a moment, the normal system. In a patient with a completely normal peripheral vestibular system, the vibration is going to stimulate the vestibular structures, but that increased neural firing reaching the vestibular nuclei is going to be symmetric. There is not going to be a provocation of nystagmus. For a normal system, we would not see nystagmus because there is no asymmetry at the vestibular nuclei. At 94%, the specificity of this test is pretty good. Similar to head shake testing, if we have a patient with bilateral vestibular dysfunction, as long as it's symmetric, you're not going to generate any asymmetry of neural firing into vestibular nuclei.
When we do see vibration-induced nystagmus, that abnormality is often going to be related to peripheral asymmetry in a unilateral dysfunction. We expect a different result from normal or bilateral symmetric if there is vestibulopathy on one side. In a case with total loss of vestibular dysfunction, we're going to stimulate both of the labyrinthine structures, and we are going to see that asymmetry because one side is not working as well as the other. That will cause the nystagmus to occur, and 91% of the time you're going to see a nystagmus that leads to the healthy side in the direction leading away from the impaired side, providing some indication of which ear is involved. If we have a total unilateral vestibular dysfunction, your sensitivity is about 98%. For partial UVD, you start to have 75% sensitivity for finding this., but the nystagmus is usually going to beat to the healthy side.
There are some instances when the nystagmus beats to the lesion side, as can be the case with Meniere's disease. But even in cases of Meniere's disease, it's going to beat to the healthy ear about 20% of the time. It often is going to beat towards the lesion side in cases of third window issues, such as Superior Canal Dehiscence. There is about an 82% sensitivity identified for Superior Canal Dehiscence with the SVINT test. Most often, the nystagmus is going to beat to the ear that has the dehiscence (i.e., to the lesion ear). Usually, we would see a horizontal nystagmus., but there is a small percentage of patients with Superior Canal Dehiscence that will have an up-beating nystagmus. The reason that we get the nystagmus beating to the lesion ear goes back to what we know about Superior Canal Dehiscence, or third window issues, and the changes in impedance that cause an increased sensitivity or bone conduction in the case of hearing, and for this vibration stimulus.
Vibration Devices Recommended by Dumas
The vibration devices recommended by Dumas include the following:
- ABC (Germany)
- ISV 1:EP500 (Amplifon, France)
- VVSED 500 (Euroclinic, Italy)
- NC70209 (North Coast Medical, USA)
- VVIB 3F (Synapsys, Marseille, France)
The one that we have been using most recently is the NC70209 from North Coast Medical in the United States. It costs about $20. If you already have video-oculography in your clinic, you should easily be able to use this device.
Contraindications & Summary
Dumas reports using this type of vibration in at least 18,500 patients, and no adverse effects were ever reported. However, he does caution clinicians to refrain from using vibration in those with recent middle ear surgery. Furthermore, if there is a history of a detached retina, subdural hematoma, or poorly controlled anti-coagulant therapy, use another form of vibration.
In summary, the SVINT test is a low-cost screening test with excellent sensitivity and specificity for peripheral vestibular asymmetry. The nystagmus will beat to the healthy ear in most cases, except Superior Canal Dehiscence and some cases of Meniere's disease where it will lead to a lesion.
A New Saccadic Indicator of Peripheral Vestibular Function Based on the Video Head Impulse Test (MacDougall et. al., 2016)
Dr. Kelsey Hatton: Before we begin our discussion, it is helpful to clarify some terminology. Head impulse testing (HIT) or head impulse paradigm (HIMP) are interchangeable terms and describe the same test. SHIMP stands for suppression head impulse paradigm and is a different test procedure.
Saccadic Health
All the testing we're covering in this paper aims to evaluate saccadic function. Evaluating saccades is important for establishing normal oculomotor function, and for the ability to drive the VOR prior to evaluating nystagmus provoked during vestibular testing.
Starting with the basic facts, saccades are high-velocity eye movements used to help us acquire moving visual targets or to maintain stable visual percept during head movements. They can be initiated very quickly. They're generally controlled by a variety of mechanisms in the brain but predominantly maintained by the cerebellum. If the head is shifted quickly to the left, a corrective eye movement occurs where the eyes rotate in their socket to maintain their target. The subject ends with their head facing to the left, but eyes directed toward the right. The key numerical fact to keep in mind is that the amount of time that passes when our eyes are trying to acquire a target with saccades is around 50 to 100 milliseconds.
Saccadic testing and head static positions are important, but not as helpful in evaluating the functional impact of VOR deficits on a patient. To get at that aspect, we care more about what happens when the system is coding for movements of the head rather than static head positions.
Oculocephalic/Doll’s Eye Reflex
A few screenings with dynamic head movements paved the way for SHIMP testing. Starting well before 1957, bedside testing observing eyes while moving the head was initially done on comatose patients. This test is also known as the Doll's Eye Reflex text. It's not a test of saccadic function, but an evaluation of the oculocephalic reflex. This is a reflex typically suppressed by the cerebral cortex, but if you are in a coma, you lose cortical inhibition. If the brain stem is functionally intact, the patient's eyes would behave like a baby doll with rotating eyes. If you had a negative doll's eye test, the eyes would stay fixed mid-orbit, and as you're moving the subjects head, the eyes don't move. This is a sign that a comatose patient's brain stem is not functionally intact and carries a poor prognosis.
Head Thrust Testing (HTT)
Head thrust testing is a similar procedure that began with testing alert patients instead of comatose patients. The main difference between the HTT and the Doll's Eye Reflex is that with the patient alert, the vestibulo-ocular reflex is the responding mechanism, with saccades as the output.
For bedside head thrust testing, you have the patient look at your nose or face and you move their head. The head acceleration should be equal to or greater than 200 degrees per second squared, in order to rule out the use of pursuit mechanisms. Because you're only evaluating saccades, you don't want to have smooth pursuit responses interfere with test performance. Typically speeds of 150-250 degrees per second squared are advocated in the literature for guaranteeing the isolation of saccadic responses.
During and after the head movement that is controlled by the examiner, the patient is meant to maintain their eyes on the target. The examiner is only able to evaluate eye movements of the patient once the head has stopped moving. If the patient is able to maintain their eyes on the target, they've successfully generated a large enough eye movement during the head movement. If the patient is unable to maintain eyes on the target, they'll have to generate the catch-up saccade after the head stops moving. This is outside of that 100-millisecond period where we would expect saccades to be activated.
While positive head thrust testing is relatively sensitive (about 71% for unilateral hypofunction and 84% for bilateral hypo-function) and specific (82%) for identifying vestibular hypofunction, there are limitations to the screening (Schubert et al., 2004). The head thrust test doesn't allow the examiner to observe eye movements that are generated by the patient during the head movements. Researchers decided they wanted to know more about what was happening during head movements. As such, the Scleral Search Coil Method was developed to monitor pupil movements. This additional research led to the development of the head impulse paradigm (HIMP).
Head Impulse Paradigm (HIT/HIMP)
The Head Impulse Paradigm (HIMP) or Head Impulse Test (HIT) was first described by Halmagyi and Curthoys in 1988. When it was first developed, it was initially performed with a scleral coil. A modified contact lens electrode was worn during head movements. Head thrust testing was performed essentially the same as it was without the lens, but the patient sat inside of a magnetic field. The contact lens electrode allowed eye movements to be measured as voltage changes in the magnetic field. They were able to identify and look more closely at those eye movements in a graphical representation. This technique was applied to patients that had abnormal bedside head thrust testing, revealing the presence of previously unobservable eye movements occurring during head movements. These abnormal eye movements needed to be differentiated from normal saccades that occur during head movements and abnormal saccades that occur after head movements. The term "covert" saccades was applied if they were hidden during the head movement, or hidden without using equipment to examine them. The term "overt" saccades refers to those that can be seen without equipment, like traditional head thrust testing.
Video-oculography. Thankfully, we no longer have to wear a contact lens with a wire in it if we want to do this test. The test administration has changed as video head impulse testing has become available. Video tracking systems advanced and were able to record enough frames per second to capture saccadic eye movements. These systems offer advantages to patients and clinicians in the form of time saved during testing, as well as added patient comfort. The goggles do require regular maintenance to keep a sufficiently tight headband because a loose fit can reduce the accuracy of the video tracking the pupil.
When you are performing the head thrust test, the subject is facing the examiner during the head impulses. When you do a head impulse test, the examiner is standing behind a seated subject. The subject is wearing goggles that have evolved over time to be smaller and lighter. The subject is about three feet away from a target on the wall that is approximately one-inch square. They have to keep their eyes on the target while the examiner is moving their head randomly and quickly. Each trial of the examiner moving the head is called a hit or an impulse. Because the head turns are administered by the examiner, they have to start and stop abruptly because it's more of an impulse than a movement.
With HIMP, examiners have to be mindful of achieving those fast head accelerations (between 150 to 250 degrees per second squared) and stopping the impulse abruptly to minimize head overshoot and eye “bounce”, in order to obtain a high-quality sample. Ambient lighting, as well as skin and hair properties can influence testing because these factors drive pupil size, video quality, and the tightness of the headband fit. Overall, video systems were validated as equally able to track the pupils compared to the scleral coil method. As such, we achieved FDA approval in 2013 for the common version of the test that people use now in clinics.
In summary, HIT or HIMP testing uses video goggles that have a high refresh rate to capture saccadic eye movements during and after quick head movements, which are applied by the examiner. Abnormal saccades would be categorized as “overt” or “covert” and suggest vestibular impairment in the canal being tested.
It should be noted that the overt and covert compensatory saccades are being added to achieve higher gain for the eye movement. They are used as an additive strategy to move the eyes further than the impaired VOR reflex thought that they should move. On any graphical representation, HIMP compensatory saccades will be in the same direction as the overall response gain. If the gain is represented as upward, the covert and overt saccades should also appear in the upward direction. Again, we're trying to push the gain of the eye movements to match what the head movement was. Normal subjects don't make compensatory saccades, because a healthy VOR attempts to execute eye movements during head movements, in order to maintain that visual fixation on the target.
The HIMP test is robust for normal subjects across the lifespan. For patients between 20 and 80 years of age, by decade, almost everyone has a gain of better than 80% with their eye movements relative to their head movements. As you see gains of less than 80%, you might expect impairment.
SHIMP
The suppression head impulse paradigm (SHIMP) test is a tweak on the HIMP test. The examiner still stands behind the seated subject who is wearing those tightly-fit video goggles. The examiner provides the impulses or random, high-acceleration head movements. However, instead of the target staying in the same place when moving the subject's head (i.e., an earth-fixed target on the wall), the target moves with the head movement (i.e., a head-fixed target). A laser mounting on the goggles will project a dot so that it moves with the head.
As we reviewed with the HIMP test, we would expect normal subjects to move their eyes reflexively in the opposite direction of the head during the head movement. In the SHIMP test, we still think normal subjects would move their eyes reflexively. Since the target has moved, they will have to make a corrective saccade in order to find the target because it's not in the same place. In normal subjects with HIMP, the eye recording looks smooth, but in normal subjects with SHIMP, there is a large corrective saccade after the head movement ends.
Patients with impairment had to engage a compensatory mechanism of extra saccades in the HIMP test to keep their eyes on the earth-fixed target. With the SHIMP test, they never had their eyes move away from the target as much as the normal subjects did, leading to weak or absent anti-compensatory saccades. Therefore, for abnormal subjects in HIMP, the eye recording shows “catch-up” saccades. For abnormal subjects in SHIMP, they do not always generate a corrective saccade after head movements and tracing looks smooth.
Proposed as complementary tests. SHIMP and HIMP are meant to be complementary tests, with SHIMP as an extension of data that you might have obtained from HIMP testing. We would expect that saccades on HIMP testing would be present if there was VOR dysfunction, whereas we would only expect saccades on SHIMP testing with VOR function.
Gain should be around 80% of eye movement relative to head movement. In this case study, the normal control subjects had the largest anti-compensatory saccades in SHIMP testing. A normal system should provoke that. SHIMP responses have lower gains than HIMP responses, with the suggested cutoff to be considered "normal" in this paper at around 66%. It's a smaller gain than what we see in Head Impulse Testing in general. If you use those numbers of normal gains for HIMP testing is 80%. If you set normal gain for SHIMP testing at 66%, then SHIMP testing is 100% sensitive and specific in separating people with unilateral weaknesses from normal subjects.
It should be noted the anti-compensatory saccades are present because the patient is trying to correct for not calculating the target appropriately. SHIMP responses have to be engaged to reverse or reduce the distance that the eyes moved. They are used as a subtractive strategy to move the eyes back to the target because the healthy VOR reflex moved the eyes too far. On any graphical representation, SHIMP anti-compensatory saccades will be in the opposite direction as overall response gain. Abnormal subjects have compensatory saccades on HIMP testing that are overt or covert. For SHIMP testing, an abnormal subject would have small, or no anti-compensatory saccades.
Benefits of SHIMP. SHIMP is another way to approach measuring the VOR response and can help clarify findings from HIMP testing that may be difficult to interpret. There is an added benefit of anti-compensatory saccades only occurring after the head movement has concluded in this test. Interpretation may be simpler when saccades are only anticipated in a small time window following the conclusion of the head movement (as opposed to during and after the head movement like in HIMP). For someone with a unilateral weakness, if they had spontaneous nystagmus, it might be difficult to interpret their HIMP testing. Their SHIMP testing would not be as affected by spontaneous nystagmus. If they have a bilateral vestibular weakness, they have responses that are covert and/or overt saccades on HIMP testing. However, you can use a SHIMP test to know whether or not they have a VOR response, or they're just using visual cues to use those saccades as correction factors.
Summary. The SHIMP is evaluating anti-compensatory saccades which are large and immediately follow the head stopping in normal subjects due to normally-functioning VOR, driving eye movements past a head-fixed target. Subjects with abnormal VOR responses don't have their eyes move as much upon the initial head thrust, and therefore, they don't make as much correction to achieve the head-fixed target. This yields small or absent anti-compensatory saccades in SHIMP testing.
References
Chiarovano, E., Vidal, P. P., Magnani, C., Lamas, G., Curthoys, I. S., & de Waele, C. (2016). Absence of rotation perception during warm water caloric irrigation in some seniors with postural instability. Frontiers in neurology, 7(4).
Dumas, G., Perrin, P., Ouedraogo, E., & Schmerber, S. (2016). How to perform the skull vibration-induced nystagmus test (SVINT). European annals of otorhinolaryngology, head and neck diseases, 133(5), 343-348.
Jacobson, G. P., Piker, E. G., Grantham, S. L., & English, L. N. (2017). Age predicts the absence of caloric-induced vertigo. Journal of Otology.
MacDougall, H. G., McGarvie, L. A., Halmagyi, G. M., Rogers, S. J., Manzari, L., Burgess, A. M., ... & Weber, K. P. (2016). A new saccadic indicator of peripheral vestibular function based on the video head impulse test. Neurology, 87(4), 410-418.
MacDougall, H. G., McGarvie, L. A., Halmagyi, G. M., Curthoys, I. S., Weber, K. P. (2013). Application of the video head impulse test to detect vertical semicircular canal dysfunction. Otol Neurotol. 34, 974-979.
Citation
Jacobson, G., Roberts, R. & Hatton, K. (2018, June). Vanderbilt audiology journal club: what's new in the clinical vestibular sciences? AudiologyOnline, Article 22152. Retrieved from https://www.audiologyonline.com