Editor’s note: This text-based course is a transcript of the webinar, Vanderbilt Audiology Journal Club: A Vestibular Update, presented by Gary Jacobson, PhD, Richard Roberts, PhD, Kelsey Hatton, AuD.
Learning Outcomes
After this course learners will be able to:
- List recent key journal articles on the topic of vestibular that have implications for audiology clinical practice.
- Describe the findings from recent key journal articles on the topic of vestibular that have implications for audiology clinical practice.
- Explain the implications for audiology clinical practice from recent key journal articles on the topic of vestibular.
Development and Preliminary Findings of the Dizziness Symptom Profile (Jacobson et al., 2018)
Dr. Gary Jacobson: My presentation is going to be a description of a new type of business questionnaire called the Dizziness Symptom Profile, or more simply DSP. I want to begin by thanking our co-investigators from James Madison University and also Mayo Clinic Rochester. The study was published recently in the journal Ear and Hearing.
Background
We begin with a statement of the problem, there are too many dizzy patients and not enough specialists to diagnose and treat them properly. It's been estimated that there were about 69 million Americans who have experienced a vestibular disorder and about 2.4 million experience chronic imbalance. Also, it's been estimated that dizziness is the primary complaint for about 2.5% of all patients seen in primary care. In fact, 45% of dizzy patients are evaluated first by primary care doctors who have little expertise in the assessment of treatment of dizziness and vertigo. It is not surprising that a large number of patients are referred by primary care to ear specialists who are unable to manage these patients in a timely fashion.
The problem. My portion of this course addresses the disparity between the numbers, especially care providers compared to the number of dizzy patients. It's clear that there are far fewer specialists than needed to manage the millions of dizzy patients. In particular, I was surprised at the number of practicing neuro-otologists, so it's about 323 in the United States. You can add to that another 50 practicing oto-neurologists, and then again, another 50 practicing neuro-ophthalmologists. The figure is roughly 425 specialists. Since there is a shortage of specialty care providers to address vertigo and dizziness, one solution might be to diagnose correctly and manage earlier these dizzy patients.
Case history. Now, some argue that the case history is the most important test that we perform. In the case history, we attempt to obtain in a short amount of time information that makes it possible for us to generate hypotheses about the sources of a patient's underlying complaints. These hypotheses are supported or rejected based on the results of the tests that form the core of the balance function assessment. However, as you know well, obtaining a case history can be pretty frustrating. It's common for patients to volunteer information that doesn't necessarily help sort out the cause of dizziness, and these patients may feel slighted if the clinician doesn't provide them with sufficient time to tell their story.
Solution. One solution to this problem would be to develop a case history device that incorporates standard diagnostic criteria for the most common dizziness diagnoses. The device would not contain any open-ended questions. The items would be developed by dizziness experts. The results of the questionnaire would be used to create a differential diagnosis. The clinician would conduct a brief history and physical examination of the patient to determine whether they agreed or disagreed with the questionnaire. For example, if the result of the DSP indicated the patient might have positional vertigo, the care provider could do the Dix-Hallpike and head-roll test and where BPPV was identified, the appropriate repositioning maneuver could be performed and the patient could be cured. If the DSP pointed toward a vestibular migraine, the clinician could place the patient on a migraine diet for 60 days and then reassess the patient with the DSP and any number of the migraine severity measures. Alternately, where the result of the DSP suggested the patient might have Meniere's disease or superior canal dehiscence syndrome, the patient would be referred directly to subspecialty care. In this way, even if the patient was not initially examined and managed by a dizziness expert, they could be evaluated against dizziness criteria developed by dizziness experts, and the initial management of the patient could be driven by care pathways developed by the same experts. I want to stress that the goal of the effort was not to diagnose disease or replace ear specialists, but instead for primary care providers, general ENTs and audiologists, to evaluate patients more efficiently by using this tool to help winnow down the number of candidate diagnoses.
To this end, we conducted three studies with over 700 subjects, and the purpose of the 1st and 2nd studies was to help us develop the DSP. The third study was conducted to determine what was the level of agreement between the differential diagnoses generated by the DSP compared to the differential diagnosis developed by the neurootologist, which served as the Gold Standard.
Investigation
The initial version of this device consisted of 64 items, and the content of the various items was based on accepted diagnostic criteria, guidelines and consensus statements, expert opinion and empirical observations obtained during case history taking obtained over decades of clinical assessments. The big difference between the DSP and other focused case histories is that instead of asking patients a series of open-ended questions, each case history item was a statement. The patients were asked to respond to each statement using a zero to four response, where zero represented a strongly disagree, a two represented a neutral response, and a four represented a strongly agree response. For example:
- There are times when I get dizzy and also have a headache. (Vestibular migraine)
- I have a roaring sound in one ear only before or during a dizziness attack. (Meniere’s syndrome)
- I have had constant dizziness for more than 3 months. (PPPD)
- I can hear my eyes move. (SSCD)
- My dizziness is intense but lasts for seconds to minutes. (BPPV)
- I had a spell of spinning dizziness that lasted for days or weeks after I had a cold or flu. (Neuritis labyrinthitis)
- I have a tingling sensation in my feet. (Multisensory system impairment)
So for each item, not only did we obtain an endorsement or lack of endorsement, but also the strength of that endorsement. The results of the study were entered into a spreadsheet where the scores of the one to 64 items were entered horizontally, and the subject numbers were entered vertically. The resulting spreadsheet was loaded into SPSS, which is a popular statistical package, and a principal components analysis was applied to the data set. Now, this is a pretty cool statistic. The PCA helps to identify items that a particular subject subgroup responded to in a similar way. Each of these subgroups of items is referred to as components, and this statistic allowed us to identify seven subgroups of items. The next step was to examine each of the subgroups of items to find the common thread running through each.
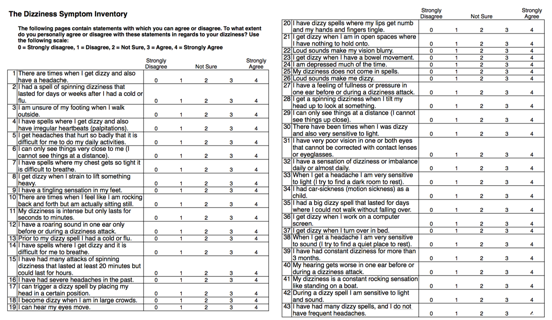

Figure 1. 64-item Dizziness Symptom Inventory randomized subject response form.
Components.
Items comprising component one described issues associated with a vestibular migraine:
- Item 53: I get headaches that hurt so badly that I am completely unable to do my daily activities
- Item 33: When I get a headache I am very sensitive to light (I try to find a dark room to rest.
- Item 57: Members of my family get migraine headaches.
- Item 16: I have had severe headaches in the past.
- Item 38: When I get a headache I am very sensitive to sound (I try to find a quiet place to rest)
- Item 46: My vision changes before a headache begins
- Item 1: There are times when I get dizzy and also have a headache
Items comprising component two described issues associated with Meniere's disease:
- Item 12: I have a roaring in one ear only before or during a dizziness attack
- Item 47: I lost hearing in one ear after an attack of spinning dizziness
- Item 40: My hearing gets worse in one ear before or during a dizziness attack
- Item 44: I hear my voice more loudly in one ear compared to the other
- Item 27: I have a feeling of fullness or pressure in one ear before or during a dizziness attack
Items comprising component three described issues associated with superior canal dehiscence (SCD):
- Item 50: I get dizzy when I sneeze.
- Item 55: When I cough I get dizzy.
- Item 22: Loud sounds make my vision blurry.
- Item 26: Loud sounds make me dizzy.
- Item 8: I get dizzy when I strain to lift something heavy.
Items comprising component four described issues associated with vestibular neuritis:
- Item 52: I have had a single severe spell of spinning dizziness that lasted days or weeks.
- Item 60: I had a single constant spell of spinning dizziness that lasted longer than 2-3 days.
- Item 35: I had a big dizzy spell that lasted for days where I could not walk without falling over
- Item 2: I had a spell of spinning dizziness that lasted for days or weeks after I had a cold or flu.
Items comprising component five described issues associated with positional vertigo (BPPV):
- Item 56: I get short-lasting, spinning dizziness that happens when I go from sitting to lying down.
- Item: 37: I get dizzy when I turn over in bed.
- Item 11: My dizziness is intense but only lasts for seconds to minutes.
- Item 17: I can trigger a dizzy spell by placing my head in a certain position.
- Item 48: I get short-lasting, spinning dizziness that happens when I bend forward to pick something up.
Items comprising component six described issues associated with Persistent, postural, perceptual dizziness (PPPD):
- Item 14: I have spells where I get dizzy, and it is difficult for me to breathe.
- Item 59: I get dizzy spells where I also feel like I have a lump in my throat.
- Item 4: I get dizzy spells where I get dizzy and also have irregular heartbeats (palpitations)
- Item 24: I am depressed much of the time
- Item 61: I am anxious much of the time
items comprising component seven described non-specific unsteadiness that we have observed in patients with utricular impairments and also in anxious patients:
- Item 58: I am unsteady on my feet all the time.
- Item 3: I am unsure of my footing when I walk outside.
- Item 21: I get dizzy when I am in open spaces and have nothing to hold on to.
- Item 32: I have a sensation of dizziness or imbalance daily or almost daily.
The phase one study permitted us to reduce the size of the questionnaire from 64 items to 35 items. For the phase two study, this 35-item version of the DSP, was administered to an additional 350 subjects to determine whether the component structure was repeatable. The result showed, again, that there were seven components identified and the items comprised the components were identical to those in the previous study. We were able to delete four additional items, reducing the DSP to a 31-item questionnaire. The third study was conducted to determine the level of agreement between the differential diagnosis suggested by the DSP and the differential diagnosis generated by the ear specialist who served as the Gold Standard.

Figure 2. 31-item DSP which was the product of the first two studies.
This is the 31 item DSP that was a product of the first two studies, it all fits on one page. The final version of the DSP consists of seven subscales with each subscale composed of three to five items each. We developed a scoring system for the DSP. The subscale scores all were normalized to 100%. In a situation where a subscale consisted of three items, with four points being the maximum score for each item, the maximum unnormalized score was 12 points. If we divided 12 into 100, we'd find that each point is worth 8.33%. In the situation where a subscale consisted of five items, each point would have been worth 5%.
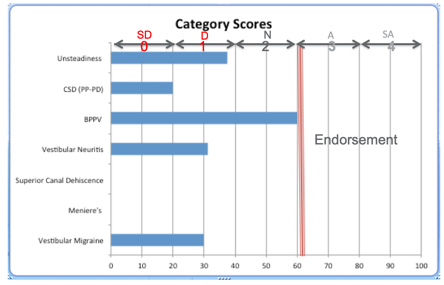
Figure 3. Patient DSP summary data sheet.
Figure 3 shows the output of the DSP. On the y-axis are the subscales we identified with the PCA, and the score from zero to 100% is presented on the x-axis. We somewhat arbitrarily said that a score of 60% or greater represented an endorsement of the item, and that value was represented by a vertical red bar in figure 3. For this patient, there was only one diagnosis that met the 60% criterion, and that was positional vertigo. A form is then completed by the neurootologist for this investigation. If the physician felt the patient's history and physical examination supported the diagnosis of PPPD and positional vertigo with PPPD predominating, they would indicate that on the physician's data collection form. They were permitted to choose up to three diagnoses for each patient, and they were asked to rank the diagnoses, with one representing the physician's top choice. This version of the DSP was given to 195 dizzy patients, and the results of this third investigation are shown on the next slide. The overall level of agreement was 70% between the DSP and the ear specialist. This level of agreement for the individual subscales where the sample size was large enough to render a judgment was between 59% for generalized unsteadiness, and 100% for a Meniere's disease, which we thought was pretty good. So the diagnoses associated with the best classification accuracy for Meniere's disease, vestibular migraine, BPPV, and generalized unsteadiness, the sample size was too small to estimate accuracy for the diagnoses of SCD, vestibular neuritis, and PPPD.
Application of DSP
So how are we using this thing? I use this device with every balance patient now. When the patient arrives for testing, I gather their self-report questionnaires, and I score the dizziness handicap inventory, the hospital anxiety and depression scale, and the DSP. The DHI provides me with an overall impact value of dizziness on the patient. The HADS tells me whether I need to be concerned that the patient's complaints may be at least partly anxiety related. The DSP helps direct me to areas I need to focus on in the assessment.
Case study. This is an illustrative example of how the DSP might be used. This is a case of a 43-year-old female with a history of severe vertigo three weeks ago, lasting days to a week. The patient was hospitalized and treated with steroids and suppressants, and now the patient reports that they are improving slowly. The audiogram was normal. There was no evidence of significant anxiety or depression, although the patient showed severe self-report dizziness handicap. The DSP suggested that the patient might have had vestibular neuritis, and further, the result of the DSP showed the patient had generalized unsteadiness. The vHIT showed abnormal function on the right side with both overt and covert saccades. The caloric test showed an absent response on the right side indicating the patient had a right-sided peripheral vestibular system impairment. The rotary chair assessment showed reduced VOR gain across frequencies. The cVEMP was normal bilaterally. The oVEMP was absent on the right side which is the same side as the caloric weakness. Remember that the DSP suggested that vestibular neuritis and generalized unsteadiness be considered in the differential and the results of the neuro-diagnostic testing supported either or both of those diagnoses. Our feeling was that the patient most likely had experienced a right superior neuritis. So what comes next? We have a series of studies ongoing to assess the test/retest reliability of the DSP. In the meantime, we are planning to field-test theDSP to obtain feedback from practitioners about its potential usefulness. If you are interested in obtaining the DSP you need only send a request to gary.jacobson@vumc.org. Of course, there will be a statement indicating that, in the end, you are, or the physician is, ultimately responsible for any diagnostic or treatment decisions you make based on the results of the DSP.
Compensatory Saccades and Head Impulse Testing Influence the Dynamic Visual Acuity of Patients with Unilateral Peripheral Vestibulopathy (Wettstein, Weber, Bockisch, & Hegemann, 2016)
Dr. Richard Roberts: I am excited to discuss this paper which is among the first to describe the relationship between test results we see on the Video Head Impulse Test (vHIT), and the Dynamic Visual Acuity test in patients with unilateral vestibular impairment. What I hope you'll notice is because of this relationship, there's an opportunity for us to gain a better understanding of the process of central vestibular compensation. Also, there is potential for clinical application to use these types of vHIT results to help determine who could benefit from vestibular rehabilitation therapy (VRT) to address vestibulo-ocular reflex dysfunction. We may also be able to use these data as an adjunct to measuring progress towards therapeutic goals and an endpoint indicator of when these goals have been met.
Background: Vestibulo-Ocular Reflex (VOR)
This particular article was published in the Journal of Vestibular Research in 2016. To understand why this article is important, let's briefly review the vestibulo-ocular reflex (VOR). This pathway exists to help us maintain visual targets on the most sensitive part of the retina, the fovea, that's the part that has the highest density of cones. That part is key for helping us to have clear image perception during head motion. We need to keep the eyes pointed at the target of interest. If our eyes don't move to mitigate the effects of natural head movements, then the target image can slip off of the fovea, and our vision is blurred. The vestibular system senses information about head movement so things like velocity, direction, head movement displacement, and that information's sent to the brainstem which then directs appropriate neural drive to the extraocular muscles. It's an incredible system that we often take for granted. All of us probably take that for granted until we experience vestibular impairment. If the vestibular area is not reacting in a healthy way because of impairment, the signals received by the brainstem, and ultimately the neural drive to the eye muscles will be inaccurate. That person will have blurred vision with the head movement, which is termed oscillopsia.
Dynamic Visual Acuity. Let's talk about a couple of ways to get an idea of VOR function. Since the vestibulo-ocular reflex relies on head movement, one thing we can do is measure visual acuity with the head still and compare that to visual acuity performance with head movement. You can do this clinically with the simple Snellen chart, but it's more common in research to measure visual acuity in units called logMAR, that's the log of the minimum angle of resolution. Regardless of the unit of measurement, when you have a person with normal vestibular function, there should be little to no difference in visual acuity when you measure performance with the head still and compare that to performance with head movement. Because of the VOR, it should be about the same. And because there's no difference between those two measures, the result will be a lower logMAR value. If you have a patient on the other hand with vestibular impairment for which they have not compensated, visual acuity performance is going to be poorer (higher logMAR). So you'll have a higher logMAR value with head movement when you compare that with visual acuity with the head still because the vestibular system's not delivering accurate information to the brainstem in that case.
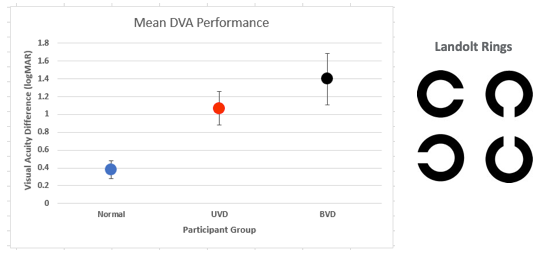
Figure 4. Common logMAR findings for three participant groups. Normal performance is <0.58 (2 SD; Vital et al., 2010).
The group with normal vestibular function over in this area has a logMAR around .38. That increases to about 1.12 for participants with unilateral vestibular dysfunction, and then the logMAR value moves up to 1.4 for the group with bilateral vestibular dysfunction. This data is actually from Vidal et al. (2010) and if we consider a two standard deviation cutoff, normal DVA should be less than .58 logMAR. These authors didn't use a Snellen chart to measure visual acuity, they actually used stimuli that you see over on the right side of this figure, called Landolt rings. For their DVA test, participants determine the direction of the opening in the ring. The size of the visual stimuli is adaptively varied and then you determine the visual acuity on logMAR. Basically, you determine the smallest Landolt ring stimuli the participant can still reliably determine the direction of the opening and then that gives you the logMAR value. It's important that we mention this because this is the exact same DVA test that was used in the Wettstein paper that we'll be talking about.
Video Head Impulse Test. Another way to assess VOR function is with the vHIT test. This test uses a high-speed camera and accelerometer coupled to the head of the patient to measure and record head movement and eye movement simultaneously. This is what many of us do in the clinic every day. By doing this, we can compare eye movement velocity and head movement velocity. Ideally, eye movement velocity should equal head movement velocity over time, which keeps the visual target maintained on the fovea of the retina. A gain value is one of the big things that we look at with this test, and that is the ratio of eye movement and head movement velocity. If eye movement velocity equals head movement velocity, our gain should be one or near one. In addition to determining Gain or VOR Gain, we also observe for Saccadic eye movements. Remember, Saccadic eye movements are very fast, non-vestibular eye movements. Those Saccadic eye movements can help move the eye towards the target when the vestibular system is not providing accurate information.
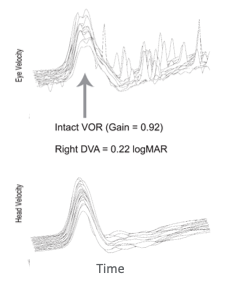
Figure 5. vHIT traces from an individual with normal vestibular function.
The top tracing of Figure 5 represents eye movement velocity across time, and then the bottom tracing represents the head movement. We can see that eye movement velocity looks very similar to head movement velocity in the lower panel. When we compare those two measures, the gain is .92, which is very close to one.
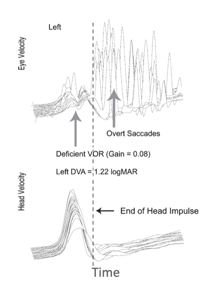
Figure 6. vHIT traces from an individual with abnormal vestibular function.
We see some striking differences in Figure 6 which show a patient with impaired vestibular function. First of all the velocity gain is much lower for eye movement compared to head movement. The VOR Gain, in this case, is .08. We also see another difference. We see some very large amplitude saccadic eye movements. This patient's saccade eye movement system is trying to get the eyes pointed towards the target of interest after the head movement. There is a hash line that's representing when the head movement impulse stops, and these eye movements are occurring after that point. Because the swift saccadic eye movements occur after the head movement, these are called overt saccades. These are the saccadic eye movements clinicians can visualize when you complete the head thrust test without any vHIT equipment. With vHIT technology though we've been able to observe there are other saccadic eye movements that can occur during the head movement. These were not able to be seen without this newer technology, and these are termed "covert saccades."
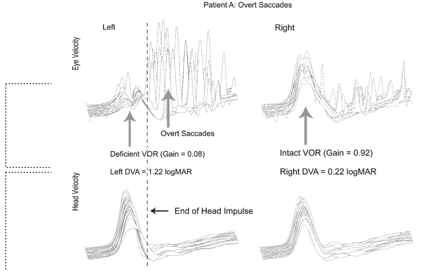
Figure 7. Overt Saccades.
In Figure 7, you can see a standard vHIT response, with a VOR Gain of .92 and almost no saccadic eye movements. With an impaired vestibular function, you see the reduced VOR gain and large amplitude overt saccades.
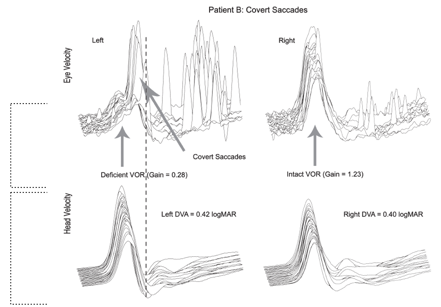
Figure 8. Covert Saccades.
The standard response on the right of Figure 8 shows a VOR Gain of 1.23, which is close to normal. But for the impaired side, here's that eye movement velocity and that results in a VOR Gain of .28 which is deficient, way less than 1.0. Now we see some overt saccades here, but they're grouped together, and they're also of an overall lower amplitude compared to the traces we just looked at. The most significant difference that we see in this figure is the presence of covert saccades. These saccadic eye movements are occurring during the head movement. The hash line represents the end of the head impulse.
What I did not show you yet is the whole basis for the study. For this patient with high amplitude, overt saccades, and no covert saccades, dynamic visual acuity (DVA) performance was poor (1.22 logMAR), which is abnormal. Vital et al. (2010) found an average DVA performance for patients with a unilateral vestibular loss was around 1.12. This performance is consistent with impairment on that side. If you look at Figure 7 at the intact side on the right, there's a logMAR value of .22, so very much normal. Looking back at Figure 8, you still have low VOR Gain and the presence of these covert saccades, and we also have lower amplitude overt saccades. DVA performance with the movement towards the impaired side for this patient is .42 logMAR. DVA performance with the movement toward the intact side is .40 logMAR. Recall that anything less than .58 logMAR is normal. So this, in fact, is what Wettstein and colleagues set out to investigate. Is there a relationship between saccadic eye movements recorded with vHIT and performance on DVA?
Measured DVA and vHIT. The authors measured DVA with the Landolt ring test that we already talked about briefly, and also with vHIT like we do every day in the clinic. They measured performance on both of the tasks for two groups of people. You had a control group with 113 folks with normal vestibular function and then a patient group that consisted of patients with permanent vestibular dysfunction. So these patients didn't have a fluctuant loss as you might observe with Meniere's disease or vestibular neuritis, they were post labyrinthectomy or post-vestibular neurectomy. They had a permanent vestibular loss.
Results
If we look at the results in the data provided in Figure 9, VOR Gain and DVA is normal for the control group. If we look at the patients with unilateral vestibular loss and we're talking about the healthy side, and then we see a different story if we look at the VOR Gain for our group or the ears of the patients in the UVL group that have an impairment, so their VOR Gain is down to .16. If we consider the dynamic visual acuity results, we have .36 logMAR for our control group, and then if we look at dynamic visual acuity for the healthy side, when the head is moved toward the healthy side in the UVL group, we are at .53 which is still less than .58 and still considered normal, but if we look at the overall dynamic visual acuity of our impaired group, or the impaired side, then we're at a logMAR of .83 which is absolutely abnormal, and that's consistent with the reduced VOR Gain on that side. So we can see that on this figure, the red data points represent the results for the affected side with the UVL patients, so they have lower VOR Gain and they're DVA performance is increased or poorer.
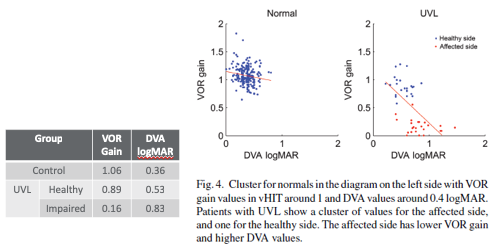
Figure 9. VOR Gain and DVA results.
The interesting result the authors of this study observed is that many of the participants with low VOR Gain have average DVA performance. The question becomes why is that present? Here's another set of figures from Wettstein et al. (2016) and these figures show the percent of head impulses, trials if you want to think about it in that way, that have covert saccades. The controls have normal DVA. And so you see the DVA score down here. And the majority of those participants had covert saccades on less than 20% of the impulses. That's not surprising since the controls can use the VOR to maintain DVA and they don't need to use covert saccadic eye movement. So we wouldn't expect them to show up for them. For participants with unilateral vestibular loss, Wettstein et al. (2016) noted an interesting relationship. As the percentage of impulses with covert saccades decreases, so as the percentage of trials with covert saccades decreases you get poorer DVA or higher logMAR. That suggests the presence of these covert saccades during the head movement is helpful with DVA performance. Recall that VOR Gain is definitely abnormal when we move towards that impaired side, so that's not helping to maintain dynamic visual acuity. The covert saccades seem to be helping position the eyes during head movement to improve or preserve dynamic visual acuity.
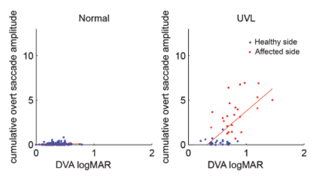
Figure 10. Overt saccade amplitude and DVA.
Figure 10 shows another pattern observed. If we look at the control group, you don't have many overt saccades, and so the cumulative overt saccade amplitude is going to be quite low. We see a similar result if we look at our unilateral vestibular loss patients for the healthy side. You see the data are in about the same position. For the impaired side, however, we do have overt saccades. Wettstein et al. (2016) observed that as overt saccade amplitude increases, then DVA and logMAR decreases. The overt saccades do not seem to be as helpful for dynamic visual acuity, but that could be an initial attempt by the central nervous system to improve dynamic visual acuity in folks that have impaired vestibular function. So those relationships between saccadic eye movement and DVA performance in patients with permanent unilateral vestibular loss and impaired VOR Gain, support that substitution of saccadic eye movements plays a prominent role in vestibular compensation.
Conclusion
There are two critical results noted by Wettstein et al. (2016), and we just mentioned those. First, DVA performance improves with the presence of covert saccades. Second, DVA performance improves with a decrease in overt saccade amplitude. There's support for the role of these saccadic eye movements regarding compensation to vestibular impairment. Remember that VOR Gain was not improving for those patients, they had a permanent vestibular loss. Something else was aiding DVA performance. Saccadic eye movements are substituting for VOR function to support visual acuity in circumstances during which the head's moving. These results may also suggest patients with uncompensated vestibular impairment follow a pattern of high amplitude overt saccades early after the impairment, followed by the emergence of covert saccades and an overall decrease in amplitude of the overt saccades. For patients who do not move completely through that process, DVA remains poor; they haven't adequately compensated. It’s vital for us to consider these results from the perspective of gaining an improved understanding of the processes underlying central vestibular compensation. From a more clinical perspective, these results could help us identify patients who need VRT, and these results may be useful in helping us track the progress of patients as they move through therapy and reach that compensation stage.
Bilateral Vestibular Weakness & Cognition
Dr. Kelsey Hatton: I will be discussing is a topic that may not have been considered five to ten years ago when we were learning about the vestibular system in our graduate programs. This opens the door to thinking about the patient in a holistic context. I wanted to discuss the association between vestibular impairments and cognition. Also, consider the effects on an individual's driving with the particular article that was chosen.
Background
Most clinicians that are performing vestibular testing understand that the otolith organs and semicircular canals are essential parts of orientation, navigation, and balance. Particularly how we sense our head movements. Often, we simplify this system when counseling patients or teaching students. We might say, "the left lateral semicircular canal lets us know when we're turning to the left or the right." While that is the primary function of that sensory organ, we know that information from all of these organs is critical to higher brain functions. Research has been illustrative in how a healthy function of these organs is important to our well-being and higher-order brain functions. Our brains are a pretty genius because they take the information about the speed of head movement from the otolith organs or semicircular canals, and break that down into more numerical values to determine speed, direction, and estimate distance. When we are receiving information from these sensory organs, we are utilizing that information to calculate the space that's around us.
Cognition. The vestibular system is also helpful in object recognition, estimating size, shape, and distance. Our vestibular system plays into the cognitive templates we create for objects, where they are in space, and recognizing objects. Dr. Roberts said we take for granted all the things that our ears, especially our balance organs contribute to as far as life functions. They're essential for navigation when we talk about estimating distance and recognizing a space. Certain brain areas have been identified as being critical gateways for incoming information from the vestibular system to the integrated relay to multisensory integration areas. The hippocampus is the main one. There is a rhythm that occurs in the hippocampus called a theta rhythm, and that is maintained by taking running information from the otolith organs and semicircular canals. (Hitier, Besnard, & Smith, 2014). You can think of the information coming from your ears, even when you're sleeping, is maintaining a rhythm. This rhythm tells us what is this space like, where am I within this space, and it's critical for us to be able to make decisions about moving around in space. The idea that there's a certain finite amount of working memory plays into this idea of if you have vestibular dysfunction there can be cognitive impairments that are not necessarily related to age but can be more directly related to changes in working memory and capacity for attention.
We know that if you have vestibular dysfunction, you can have deficits in learning new patterns or recalling patterns. Remembering sequences or processing visual information is changed and the reaction time is changed with a certain amount of vestibular dysfunction. A bottom-up change occurs when you reduce the resources concerning information from your inner ears. Then there's also a top-down change that you can have because you have less ability to make changes to keep postural stability. Reaction time is being influenced by vestibular weakness. It makes you slower at reacting, and it makes you more unstable. Researchers have shown in a variety of papers there are postural difficulties related to vestibular weakness and also postural difficulties related to memory impairments (Smith et al., 2018; Wei & Agarwal, 2017). This is important for us to consider the impact on our cognitive resources when we have a vestibular weakness because it can change your overall stability and mobility. It has been shown that patients that have vestibular dysfunction, both otolith only and canal only dysfunctions, can have increased odds ratios when it comes to tasks of concentration and memory (Smith et al., 2018; Wei & Agarwal, 2017).
Bilateral Weakness & Driving
A task that requires a lot of cognitive resources and attention is driving. We know that vestibular impairment affects memory, attention and visual processing. You can start to see breakdowns with an individual's performance as far as staying in the lane, changing lanes, and estimating where you are. It's challenging if you have a deficit in information coming from head movements. This is because you're checking you're mirrors, trying to determine how far the person in front of you is, and you're using the sensation of acceleration or braking. Those signals help you navigate when driving. There are spatial deficits that occur with vestibular impairment, and specifically affecting these components of driving. When reading, comprehension, and accuracy is lowered, being able to pull in and out of a space without hitting something is not so good. Also, checking blind spots is difficult if you have a vestibular impairment. So, when you see patients in the clinic, you want to remember that you're not just counseling them on the physical deficits that the testing is giving you. You want to think about what is the whole picture with how these deficits impair their life and their activities of daily living.
Driving is a critical topic we should be discussing with our patients. There are indeed patients that have arrived in the clinic and driven themselves where it caused great concern. If you give them paper and pen tests, their visuospatial cognitive performance can be predictive of on-road driving performance. The intersecting pentagon drawing, clock face drawing, and block design tests can be helpful in knowing if they're having trouble with judging space and representations of space. Patients that have chronic vestibulopathy, Meniere's disease or acoustic neuroma resection have a higher likelihood of reporting difficulty driving.
Vestibular Dysfunction and Difficulty with Driving: Data from the 2001–2004 National Health and Nutrition Examination Surveys (Wei & Agrawal, 2017)
The paper discusses the National Health and Nutrition Examination Survey (NHANES), which is a national survey where they validated the questions that are related to dizziness and imbalance. It helps to identify what proportion of the American population suffers from bilateral weaknesses. They used the same survey and had the subjects receive a variety of tests including visual and postural examinations as well as a general physical exam. Patients did a short postural screening which nonspecific to vestibular weakness. The screening can tell you about postural issues where they have the patient stand with eyes open on a firm surface, eyes closed on a firm surface, eyes open on foam and eyes closed on foam. They categorized if patients were unable to complete the eyes closed on foam surface task. This was the group that researchers wanted to see how did they answer the questionnaire. Did they answer the questions in alignment that had indicated bilateral weakness? The questions that were of interest were:
- “During the past 12 months have you had dizziness or difficulty with balance?”
- “During the past 12 months have you had difficulty with falling?”
- “How much difficulty do you have driving during daytime in familiar places?”
What they found:
- Driving difficulty increased significantly with increased age, poorer visual acuity, positive report of falls, and vestibular dysfunction groups
- Vestibular dysfunction via questionnaire associated with 1.47x increased odds of driving difficulty
- Vestibular dysfunction on the questionnaire and during in-person physical on condition four had 4.3x increased odd of driving difficulty
- Every additional second of holding condition 4 decreased odds of driving difficulty
- Report of driving difficulty via questionnaire associated with 5.38x higher odds of falling
- Vestibular dysfunction in clinic and report of driving difficulty associated with a 13.01x increased odds of falling compared to those denying driving difficulty
44% of adults reporting bilateral vestibular weakness via survey reported stopping driving or changing driving habits. Those with better posture leads us to believe there's more of these cognitive components that are still intact. Even with vestibular weakness this is allowing you to drive more safely. Report of driving difficulty on the questionnaire was associated with a higher risk of falling. I think it's critical for us to think about asking how people are doing with their driving. Expand our questions past our common vestibular questions because there's something related to postural control, falling and driving when we look at questionnaires like this. Let us begin to think in a bigger picture about how vestibular impairment can affect our patients. I would encourage you if you are unfamiliar with the vestibular and cognitive system, this is a great paper that summarizes the brain structures that are part of receiving information directly from the vestibular organs to make decisions about the sense of self, center, visual pathways, spatial navigation, and lots of really fascinating things.
References
Hitier, M., Besnard, S., & Smith, P. F. (2014). Vestibular pathways involved in cognition. Frontiers in integrative neuroscience, 8, 59.
Jacobson, G. P., Piker, E. G., Hatton, K., Watford, K. E., Trone, T., McCaslin, D. L., ... & Roberts, R. A. (2018). Development and preliminary findings of the dizziness symptom profile. Ear and hearing.
Smith L, Wilkinson D, Bodani M, Bicknell R, Surenthiran SS. (2018) “Short-term memory impairment in vestibular patients can arise independently of psychiatric impairment, fatigue, and sleeplessness.” Neuropsychol. 19. doi: 10.1111/jnp.12157. [Epub ahead of print] PMID:29673069
Vital, D., Hegemann, S. C., Straumann, D., Bergamin, O., Bockisch, C. J., Angehrn, D., ... & Probst, R. (2010). A new dynamic visual acuity test to assess peripheral vestibular function. Archives of Otolaryngology–Head & Neck Surgery, 136(7), 686-691.
Wei, E. X., & Agrawal, Y. (2017). Vestibular Dysfunction and Difficulty with Driving: Data from the 2001–2004 national health and nutrition examination surveys. Frontiers in neurology, 8, 557.
Wettstein, V. G., Weber, K. P., Bockisch, C. J., & Hegemann, S. C. (2016). Compensatory saccades in head impulse testing influence the dynamic visual acuity of patients with unilateral peripheral vestibulopathy 1. Journal of Vestibular Research, 26(4), 395-402.
Citation
Jacobson, G., Roberts, R. & Hatton, K. (2019). Vanderbilt audiology journal club: a vestibular update. AudiologyOnline, Article 24306. Retrieved from https://www.audiologyonline.com




