Editor’s note: This text-based course is a transcript of the webinar, Through the PSAP Looking Glass, presented by A.U. Bankaitis, PhD, FAAA .
Learning Outcomes
After this course, learners will be able to:
- Explain how to objectively evaluate device performance capabilities and corresponding appropriateness for a given audiometric configuration.
- Describe two residual limitations of PSAPs objectively verified to meet NAL-NL2 targets for a given audiometric configuration.
- List three potential PSAP distribution models for clinical practice.
Introduction
About three years ago, a couple of PSAPs came across my desk while at work with a note to the effect of "what do you think about these, and should we put it in our catalog?" That was the day that I embarked on a journey to objectively evaluate PSAPs with the help of my colleagues at Wash University Medical Center in St Louis and the University of Pittsburgh Medical Center. The primary purpose of this webinar is to share PSAP data that has been collected over the past three years, including a more detailed dissection of individual devices that have been trending on social media. I want to offer insights for whatever they may be worth your consideration based on my journey.
The content of this webinar is organized as follows. We will begin by defining and giving a general categorization of PSAPs. With this foundation, I will provide a general summary of PSAP versus hearing instrument comparison studies, which will serve as a framework for the third objective, to outline a specific PSAP vetting procedure that I have implemented to make more informed decisions about the potential viability of a device as an alternative amplification solution. I would like to then review performance data on a handful of vetted PSAPs, and what that data means in terms of potential benefit to patients. Then finally, finish up with a discussion of possible PSAP distribution models, along with their general pros and cons for consideration in your practice.
PSAP Overview
What is A PSAP?
Let's start with just a general PSAP overview. PSAPs, or personal sound amplification products are devices that are designed to amplify sounds in specific environments and situations. You often hear these "situations" defined as hunting, bird watching, and lectures. Essentially, where somebody with normal hearing may need help to hear these types of sounds. As an emerging market, there has been quite a steady influx of commercially-available PSAPs with new products being consistently and constantly introduced, as others are being discontinued and no longer available for any number of reasons. They do come in a lot of shapes and sizes, ranging anywhere from a smartphone app (i.e., EarMachine), all the way to a body-worn device (i.e., Pocket Talker). Although the majority are going to be ear level devices, some of these ear-level devices can look "quasi-futuristic," like the Etymotic Bean, or the QLeaf Pro. Most tend to resemble hearing aids like the PLAID and the E33 as you can see in Figure 1.

Figure 1. Examples of PSAPs.
How is A PSAP Different From A Hearing Instrument?
Hearing instruments. These are medical devices that are prescribed and dispensed by licensed professionals, and they are explicitly designed as a treatment for hearing loss for both adults and children. These devices are effective for degrees of hearing loss that vary anywhere from mild to severe.
OTC. In contrast, we have over-the-counter hearing aids or OTC hearing aids. This is a new class of hearing aids intended for hearing loss that is going to be available without prescription or professional intervention for adults only in the near future. These devices are for those with perceived mild to moderate hearing loss.
PSAP. These are electronic devices that are intended for situational listening, providing amplification of environmental sounds for adults with normal hearing. PSAPs are not intended for the treatment of hearing loss, although earlier published literature has generally concluded that some may be appropriate for mild to moderate hearing loss. Although more recent research has found that perhaps they are limited to very mild to mild hearing loss only.
Hearables. Then finally we have hearables, which are ear level consumer electronic devices mainly offering connectivity and designed primarily for health or entertainment-related purposes, like monitoring your heart rate, tracking steps, listening to music although some models integrate an amplification feature.
Figure 2 shows a pretty clear-cut difference amongst these devices; in reality, things are not always that straightforward. If you ignore claims of intended use on product packaging, when evaluating these devices, there can be a lot of overlap in terms of function and aspects of performance amongst these different devices. It has been my impression that there are many instances where some PSAPs are hearing instruments that have merely been repackaged and sold as PSAPs.
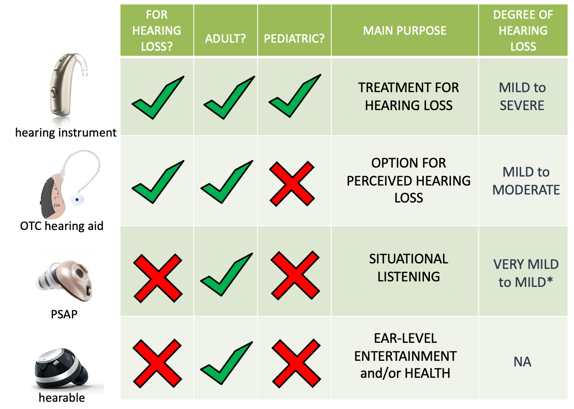
Figure 2. Comparison of PSAP vs. Hearing Instrument.
PSAP vs. HA
PLAID. Manufactured by Ear Technology and is a device that is packaged and marketed as a PSAP. The PLAID is preloaded with three amplification settings, so when you turn it on it defaults to the first setting, which offers the lowest amount of amplification. If you want to advance to the next level of amplification, you press and quickly release the upper portion of this rocker switch. The user is going to hear two beeps indicating that the second setting has been accessed. If you press and release again, the user will then hear three beeps indicating that the third or the loudest amplification setting has been activated. Now the user can continue to toggle through these settings, but then you can lock in the preferred setting and making it a new default. This is done by simply pressing and holding that rocker switch button for at least six seconds or until you hear a long beep. Now, every time that the PLAID is turned on, it's going to default to whatever that amplification setting has been. Once you do that by clicking on the upper button, the user can toggle between two modes, which are quiet and noisy environments. The rocker switch turns into a volume control wheel.
CliKEZ. The very same company offers a device that is called the CliKEZ, which you see here pictured on the right of Figure 3. It looks, and it functions exactly like the PLAID PSAP with the main difference being that the CliKEZ is a hearing aid. So the packaging material states that "it is not an over-the-counter hearing aid, internet or mail-order hearing aid." Instead, this is a hearing aid that is going to be available through healthcare professionals only that comes preloaded with the very same three amplification settings. They state that there's an advantage because it eliminates the need for the healthcare professional to connect to a device or a computer. So the CliKEZ seen here at the bottom is a hearing aid that is distributed through professional channels only. The PLAID is a PSAP also distributed through professional channels only.
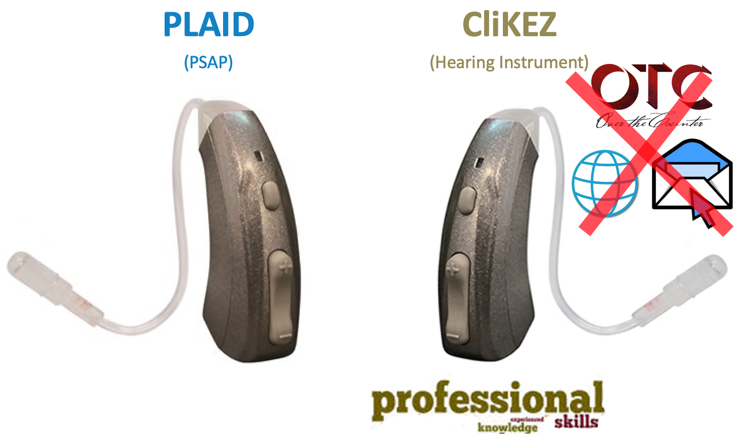
Figure 3. PLAID and ClicKEZ.
Tweak. There's a third device called the Tweak which also is a PSAP offered from the same company that looks like and functions exactly like the other two with the exception that the Tweak is a direct-to-consumer version of the PLAID. So a consumer can buy the Tweak on Amazon whereas they would have to go to a dispensing professional to get the PLAID even though they are the same device.
Etymotic Bean. The same thing happened with the Etymotic Bean. About five years ago, Etymotic introduced the PSAP called the Bean and initially, it was made available through professional channels only as well as on the direct to consumer portion of their website. The device slowly began to gain popularity on Amazon.
QSA. So as a result, the Bean was repackaged as a direct-to-consumer PSAP, and the company rolled out a new PSAP that was called the QSA which was available through professionals only. The QSA was nothing more than the Bean repackaged as a PSAP for a different distribution channel.
I bring this up not as a criticism of either company, but I intend to shine a little spotlight on the fact that there can be a lot of overlap between and amongst devices that are labeled as PSAPs. It is not only confusing for us but very confusing for our patients as well as the consumer. This is just something to keep in the back of your mind.
PSAP Categories
Retail price. So now let's talk about PSAP device categories. Over the past five years, I have seen PSAPs priced as low as $20 and as high as $799 for one unit. Although most are going to fall within the $150 to $300 range for one unit. Now earlier research initially started categorizing PSAPs according to their retail price. For example, Smith, Wilber, and Cavitt (2016) described PSAPs as either low-end or high-end. They associated an end-user price tag of less than $50 as low-end and $150 to $300 range as high-end. Reed et al. (2015) applied the same approach in their comparative studies of PSAP versus hearing instruments. Where they categorized it as a low end versus high end according to the retail price. There is certainly nothing wrong with this approach. During my first PSAP project with Washington University three years ago, we examined the performance of basic and premium hearing instruments to that of about a dozen different PSAPs; we ended up categorizing PSAPs differently.
Features. The price tag of premium versus basic hearing instruments differs considerably because premium hearing instruments offer more advanced technology and more features. We decided to approach PSAPs not from a function of the retail price but according to available PSAP features. In doing so, we found that the retail price was not a very good indicator of PSAP functionality. This is because some of the lower level devices that simply had an on/off switch, and not even a volume control ended up costing two times more than more advanced PSAPs. As a result, we came up with the three PSAP categorization that includes basic, intermediate, and advanced.
Basic. This is an out of the box type of PSAP that is going to be equipped with either a volume control or switch that is going to toggle between preset programs. The Etymotic Bean is a perfect example of a basic PSAP. It comes with a switch whereby in one position it offers 15 dB of amplification, and the other it offers 23 dB or T-coil depending on which model you buy.
Intermediate. This level of PSAPs are semi-adjustable by the user, and they come equipped with both a volume control with access to different programs like we saw in the PLAID.
Advanced. Then finally, we have a category we call advanced. These are semi-adjustable, usually using a smartphone app that allows the user not only to adjust the volume but to perform a degree of frequency shaping. In most cases, advanced PSAPs are rechargeable rather than running on hearing batteries.
PSAP vs. Hearing Aid Performance
Now that we have had a brief overview of PSAPs let's take a look at the PSAP versus hearing aid performance data. I am going to give you a general summary of the published literature. For those interested in more detail, there is a two-part PSAP series available through AudiologyOnline that goes in a lot more detail. Part one details comparison studies between PSAPs, basic and premium hearing instruments, including user preferences as well as looking at coupler measurements and device fitting capabilities, and matching prescribed targets. Part two is an extension of part one, and it starts diving into service delivery models such as first-fit algorithms versus verified program fittings and the discussion of best practice guidelines.
- PSAPs: Our Role in this Disruptive Environment - Regulatory Issues and Research Findings
- PSAPs: Our Role in this Disruptive Environment - Clinical and Business Practice Issues
For this webinar, three aspects of PSAP versus hearing instrument performance will be summarized. We will include electroacoustic performance via coupler measurements, device capabilities in meeting prescribed targets across standard audiometric profiles via probe-mic measurements and looking at outcomes like sound quality, speech performance, and noise and listening effort.
Electroacoustic Performance
In terms of electroacoustic performance, a decade's worth of product reviews and recently published comparison studies show that the electroacoustic characteristics of PSAPs vary significantly for those devices packaged with manufacturer's specification data which is rare. Measured coupler data seems to match the manufacturer's specs a lot of the time but not always. In the first phase of our project, a handful of PSAPs performed weaker than packaged specs (Voss, Oeding, Bankaitis, Pumford & Valente, 2017). Other publications and observations that we saw during our phase two project also show that some PSAPs produce MAX OSPL90 outputs that exceed 120 dB SPL (Chan & McPherson, 2015). Some were as high as 130 dB SPL, which is problematic. Given the fact that hearing aid manufacturer specifications often recommend a max tolerance no greater than 120 DB SPL for adults with mild to moderate age-related hearing loss. PSAPs tend to overamplify the low frequencies while offering minimal usable high-frequency gain (Reed, Betz, Grabowski, Korczak, Lin, & Mamo 2015; Smith, Wilbur & Cavitt, 2016; Brody, Wu & Stangl, 2018). Or they do not amplify across a broad enough frequency range particularly for those devices that have lower price points. Finally, research has shown that PSAPs tend to generate high equivalent input noise. Generally in the 40% range which exceeds the 28% tolerances for hearing instruments (Callaway & Punch, 2008; Smith, Wilbur & Cavitt, 2016; Voss, Oeding, Bankaitis, Pumford & Valente, 2017).
Probe-Mic Fitting Capabilities
A handful of published studies compared real ear aided responses of PSAPs and hearing instruments in terms of device ability to match NAL-NL2 targets across the frequency range of 250-6000 Hz using multiple input levels. The purpose of doing this was to document whether a particular device can provide the necessary output or gain for a given degree of hearing loss and corresponding audiometric configuration. While the general protocols across these studies were pretty similar, the established tolerances defining when a target was met was not consistent. In some cases, NAL-NL2 targets were considered matched if the measured REAR was within -5 to +3 dB (Callaway & Punch, 2008). In other cases, there was a more liberal tolerance of plus or minus 10 dB, while we applied a plus or minus five dB tolerance (Smith, Wilbur & Cavitt, 2016; Voss, Oeding, Bankaitis, Pumford & Valente, 2017). It's important to realize that more liberal tolerances will not only qualify a larger pool of PSAPs as meeting targets. Therefore conclude device appropriateness for a given audiometric configuration more than a stringent one. It also runs the risk of potentially overstating device capabilities in providing the necessary output for those with a particular degree of loss. While we tested some of the same devices that Smith, Wilbur & Cavitt (2016) did, this is the very reason why their study concluded that PSAPs might be appropriate for up to a moderate hearing loss. Whereas our recent research using a more stringent tolerance found that PSAPs are only viable for up to a mild hearing loss.
Sound Quality & Speech in Noise
A handful of years ago, researchers conducted laboratory comparisons of PSAPs versus both basic and premium hearing instruments in a group of adults with mild to moderate hearing loss (Cox, 2014; Cox, Johnson, & Xu, 2014; Xu, Johnson, Cox & Breitbart, 2015). No device preference was found for music or everyday noises. Although participants did prefer the sound quality of speech, with both basic and premium hearing instruments over PSAPs. Reed, Betz, Grabowski, Korczak, Lin & Mamo (2015) collected data from a small pool of five subjects. They were exploring speech and noise performance between two PSAPs versus premium hearing instrument technology. Their preliminary results on the QuickSIN and the worded noise test seemed to be the same amongst the devices which warranted further exploration. They followed up with the comparison of unaided versus aided performance on the AZ Bio sentence test. They used five PSAPs and one premium hearing aid in a group of 40 adults with mild to moderate hearing loss (Reed, Betz, Kendig, Korczak & Lin, 2017). Except for only one of the devices, PSAPs were described as associated with improvement in speech understanding for individuals with hearing loss that were similar to results that were obtained with a hearing aid.
At this time, it's critical to recognize the fact that all of the mentioned studies comparing some aspect of PSAP versus hearing instrument performance fit participants with devices that were programmed and verified using real ear measurements. Hearing instruments as well as PSAPs were fit using audiology best practice protocols. While this methodology controls for device setting variability, it does limit the generalizability of the results. In my opinion, this has often been either overlooked or ignored. PSAPs and OTC are an off-the-shelf, out-of-the-box in the ear transaction that is designed to bypass the audiologist. Therefore access to and the benefit of a verified fit is not part of the package deal. The methodologies of most comparison studies fit PSAPs using audiology best practices. I can appreciate and understand the need to do this from a research design perspective. In my opinion, the PSAP performance data has been taken out of context within our industry. The data has been taken outside of the confines of our industry, resulting in a lot of misunderstanding, misleading, and overstated headlines as to the possible true potential of these devices.
The impact of the professional component must constantly be reiterated, because it can be easily overlooked which is why I was so happy to see the most recent research article by Valente, Oeding, Brockmeyer, Smith & Kallogjieri (2018). Word, phoneme, and sentence recognition in quiet versus noise and subjective outcomes between hearing instruments that were set to manufacturer's first fit versus programmed to NAL-NL2 using real ear measurements were compared. In this double-blind, randomized controlled trial, statistically significant improvements in favor of real-ear protocols over manufacturer first fit regarding word, and phoneme recognition for soft sounds and average sounds and quiet were found. On top of that, nearly 80% of the participants preferred the programmed fit over the manufacturer's first fit. What this shows is we achieve better outcomes with devices that are programmed using real ear measurements over the very same devices that are programmed to a manufacturer's first fit. If we apply this back to PSAPs and OTC hearing aids, when study methodologies fit PSAPs or OTC using real ear measurements, the devices have been adjusted to their maximum potential in a lab setting which will not necessarily correspond to the end user's self-adjusted fitting in the real world.
We need more studies to look at hearing instruments that are fit using evidence-based best practice protocols versus PSAPs that have been self-adjusted by their participants. This is what Brody, Wu, and Stangl (2018) did in their research article that was recently published. They looked at the performance outcomes in a group of adults that were fit with a hearing instrument that was verified with real-ear measurements versus three different PSAPs that were self-adjusted by participants according to their personal preference. Although sound quality differences were minimal, hearing instruments fit using best practice verification protocols provided more audibility, better speech recognition performance, and lower listening effort compared to all PSAPs.
Coupler measurements. The PSAP literature has shown that PSAP performance ranges anywhere from being too weak or excessively loud. There's a tendency to overamplify the lows, and they are associated with producing pretty high equivalent input noise.
Probe-mic measurements. In terms of probe-mic measurements, hearing instruments match NAL-NL2 targets across a broader range of audiometric configurations. There may be some PSAPs that are potentially appropriate for individuals with hearing loss, but it's not the mild-to-moderate that was initially reported. The more recent research indicates that PSAPs may be suitable for adults up to a mild hearing loss at best. In terms of ability to meet prescribed real-ear targets, PSAP vs. hearing aid probe-mic performance data suggests hearing aids outperform PSAPs overall. Based on the most current research, when criteria for meeting NAL-NL2 targets is defined as within +/- 5 dB, the greatest degree of hearing loss some PSAPs could potentially accommodate is up to a mild hearing loss.
Speech data. The data is limited, and more research is needed in that area to get a better idea as to what is going on.
Objective Evaluation of PSAP Performance
In collaborating with Wash University and the University of Pittsburgh, I have used the same protocols that we used in our studies to vet different PSAPs that have come across my desk. This helps determine whether or not a particular device is capable of generating the necessary outputs to potentially benefit individuals with perceived mild to moderate hearing loss. Also, determining if we can offer it as a budget-friendly option to those who cannot afford traditional amplification.
Now, is this PSAP vetting process that I'm going to show you a good one or even appropriate? I don't know. Will it need to be modified? It already has, as I've been learning a little bit more and it's likely going to change. It's not perfect. What I do know is it works off of a knowledge base familiar to me, and my hearing industry colleagues and without having anything else in place, I feel that it's better than nothing. On a personal level, once I started vetting PSAPs according to this protocol, I felt more comfortable making judgment calls and recommendations to my colleagues than I did without it. This vetting process can be summed up in three necessary test steps; subjective assessment (listening check), electroacoustic analysis and, probe-mic measurements.
Subjective Assessment
PSAPs that come across my desk are evaluated in terms of ease of use and offered features. A listening check is performed to assess the sound quality and also to get a feel for how the device performs while manipulating available controls, like volume control or a program switch.
Electroacoustic Analysis
While many PSAPs are not packaged with manufacturer's specification data, running devices on a test box provides insight on device quality. This also examines whether or not it's going to be suitable for those with mild to moderate hearing loss. During coupler measurements, the appropriate couplers are used depending on if the PSAP is a RIC, custom or a BTE. PSAPs are then run in a hearing aid test box using the ANSI 2009 standard. We are capturing everything (i.e., Max OSPL90, total harmonic distortion, equivalent input noise). While traditional hearing instruments ran in a test box are adjusted to full-on gain and reference test gain appropriately, PSAPs, do not have a lot of room to work with. Thus these devices are set to the maximum gain setting throughout all coupler measurements. Whether that means the highest volume control setting that is available, a program with the most significant gain or the program with the greatest gain at the highest volume control setting available. This is how we perform the electroacoustic analysis. To reiterate, PSAP specification data is not included in the packaging, and it's just a matter of looking at things to make a judgment call in terms of coupler measurements. Finally, laboratory REAR measurements are used to determine which NAL-NL2 targets a PSAP can best match across nine audiometric frequencies ranging between 250 and 6,000 Hz for a given audiometric configuration using the international speech test signal that is presented at soft and average speech levels only.
Standard Audiometric Configurations
Eight of the ten standard audiograms defined in the International Electrotechnical Commission standard are used to assess device capability. The eight standard audiograms show in Figure 4 represent mild to severe degrees of hearing loss that either has a flat, moderately sloping or steeply sloping configuration. I want to take a moment to look at each of these standard audiograms individually because as we look at PSAP performance data, these audiograms are going to come back up.
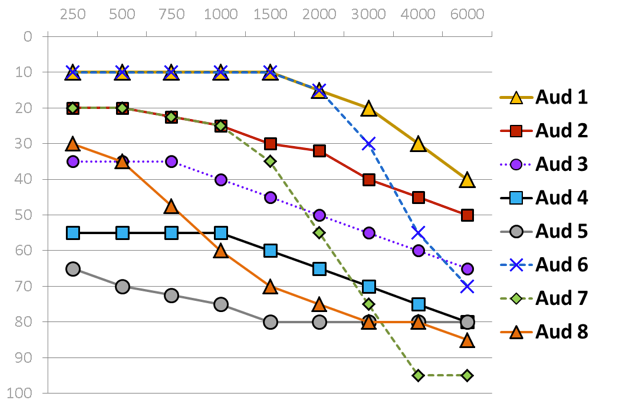
Figure 4. Standard audiometric configurations.
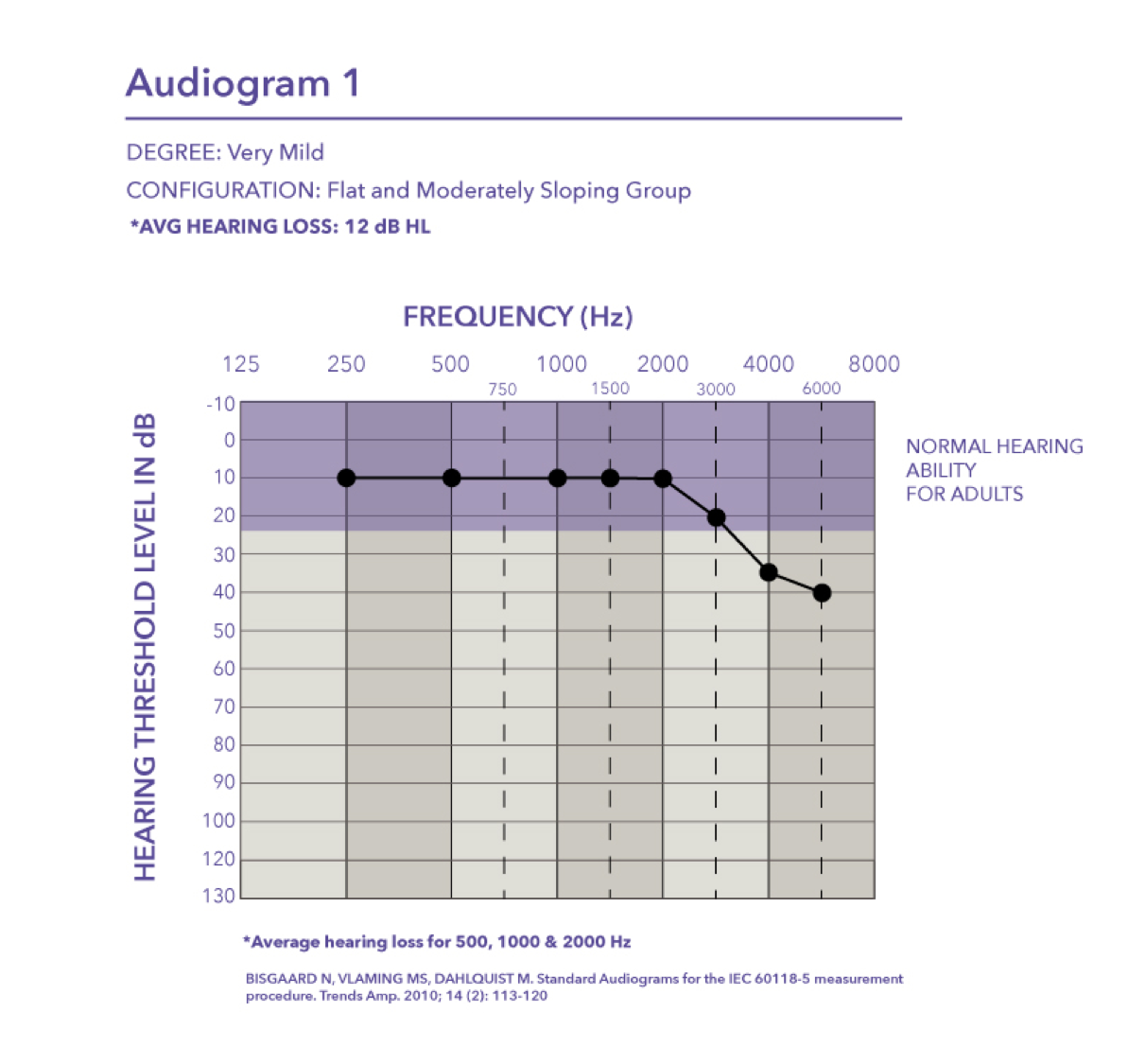
Very mild. It falls into the flat and moderately sloping group of audiometric configurations. The critical thing to keep in mind is when you look at the pure tone average of audiogram one, the PTA is basically 12 dB HL. The majority of the hearing loss is in the high frequencies starting at 4000 Hz.
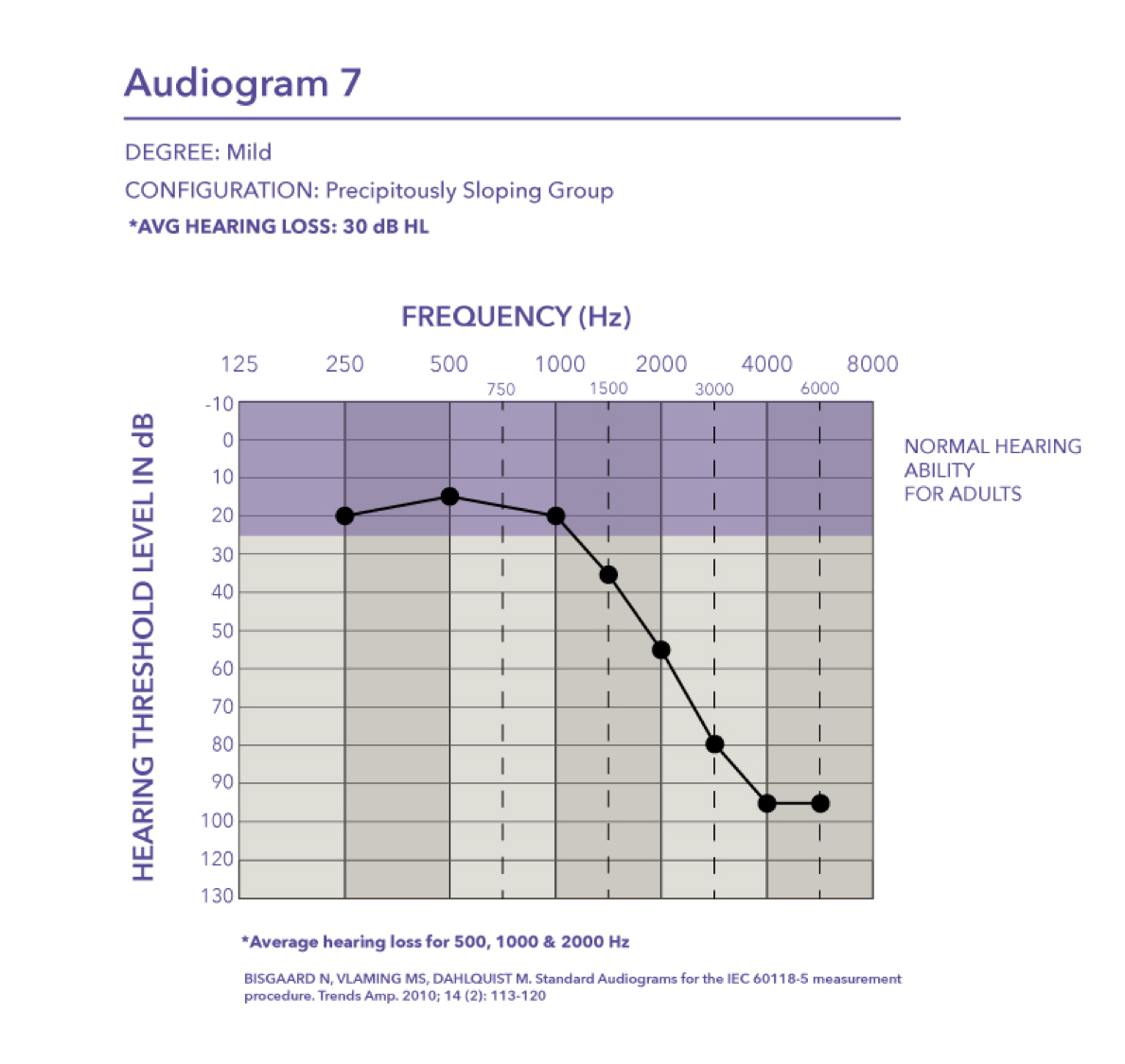
Mild. Audiogram two is also a mild hearing loss where the pure tone average is 27 dB HL.
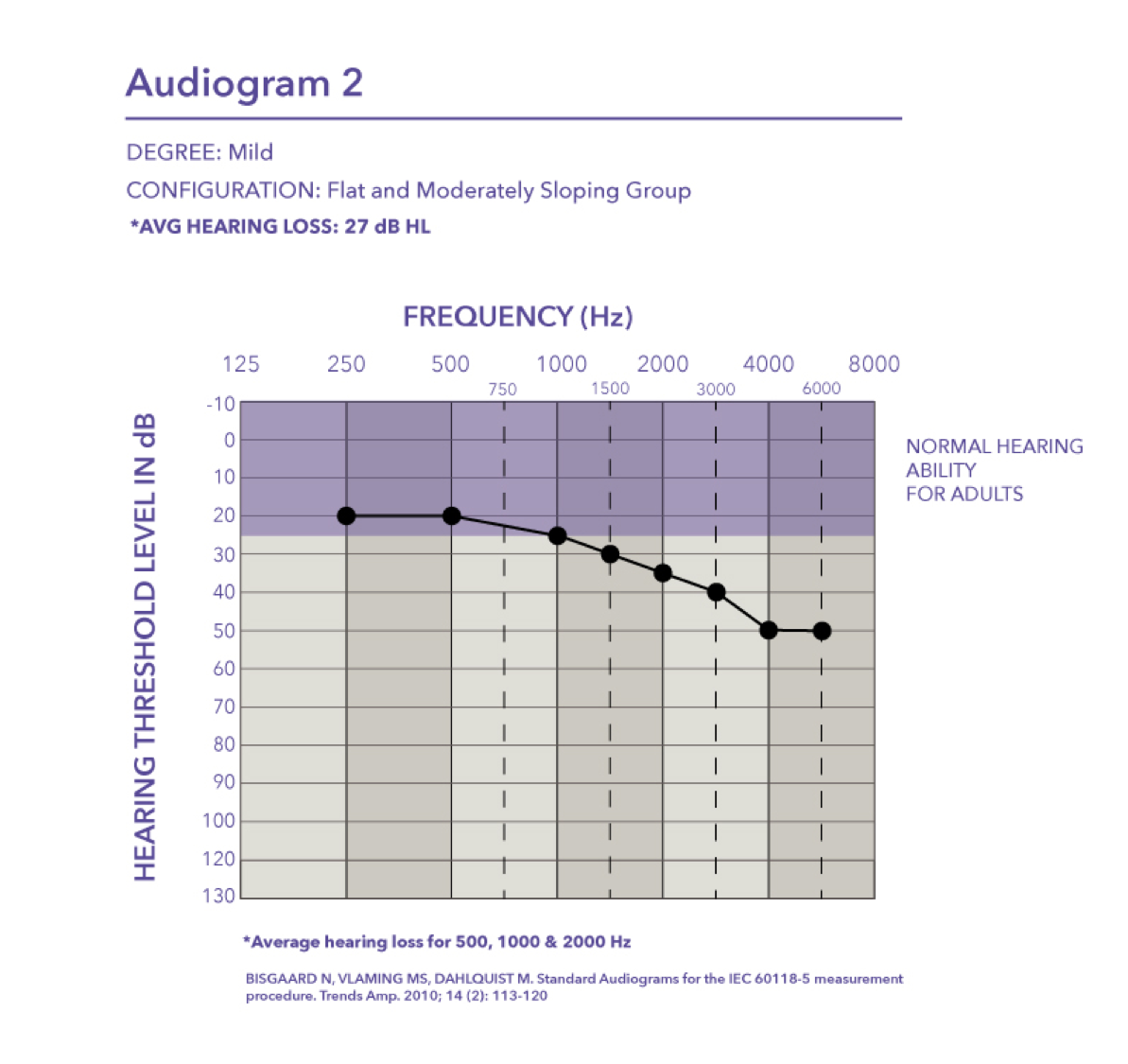
Moderate. We have audiogram three, which is approaching a moderate loss with the pure tone average of 42 dB HL.
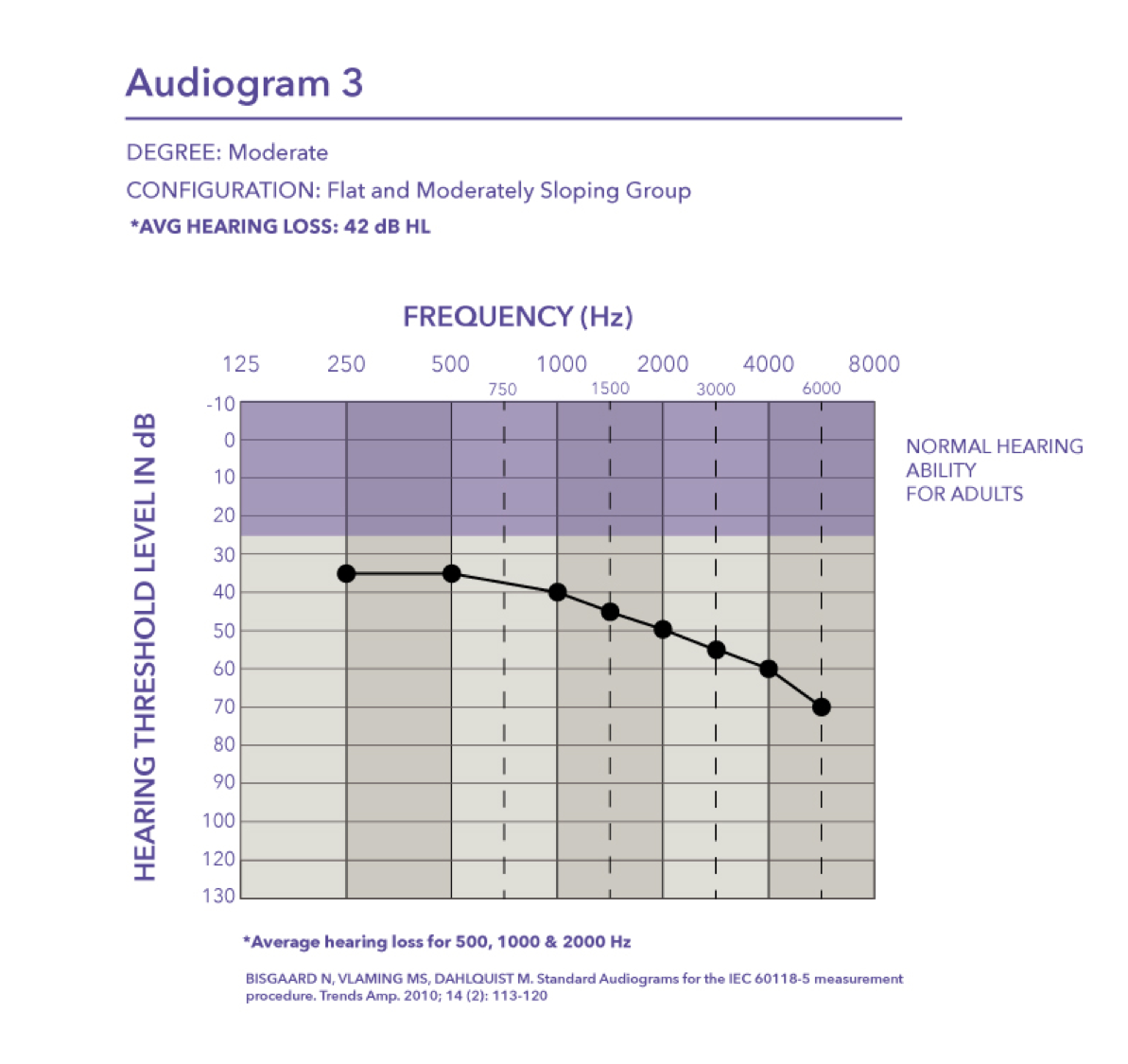
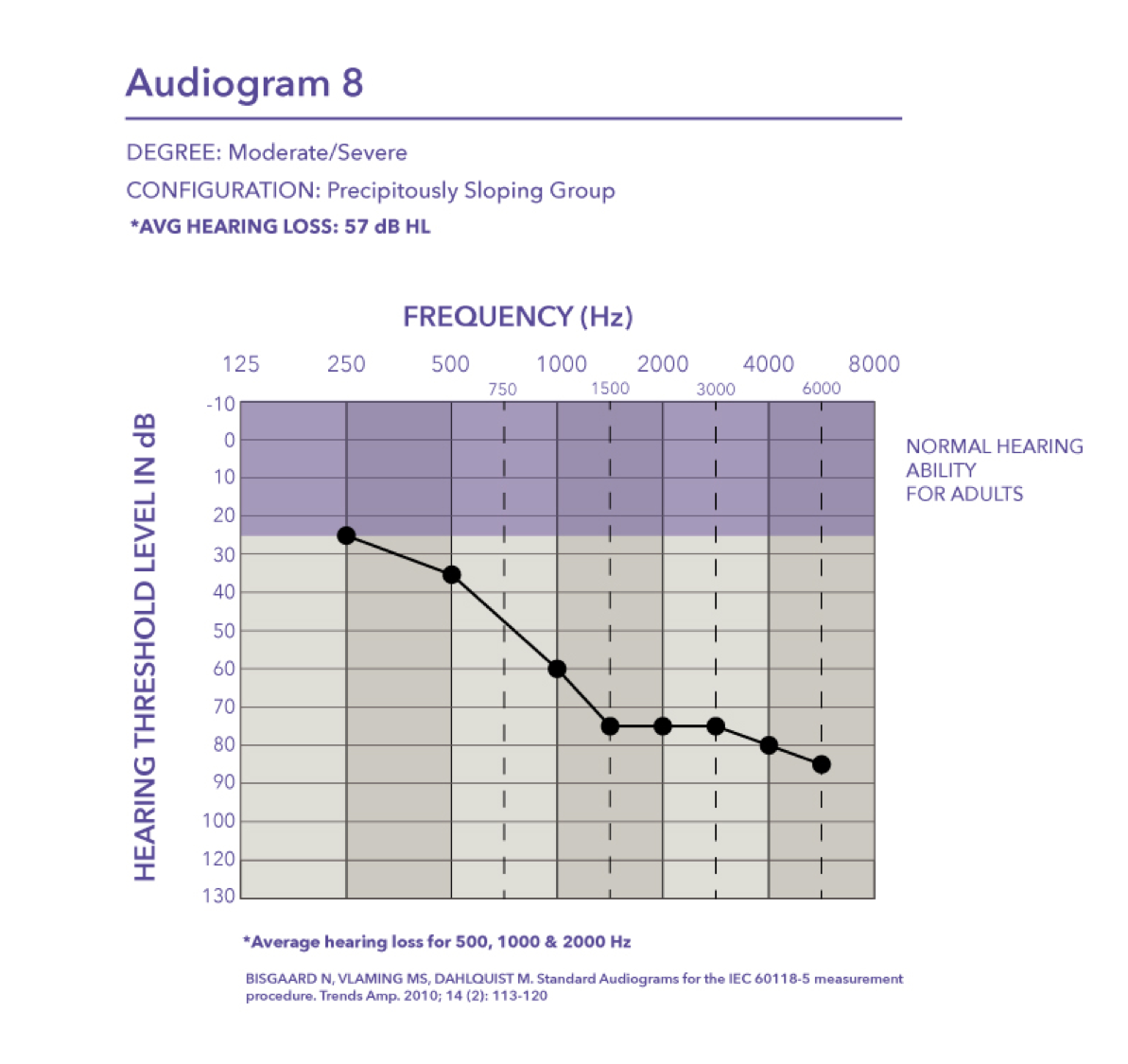
Moderate/Severe. Audiogram four is a moderate to severe hearing loss with a pure tone average of 58 dB HL.
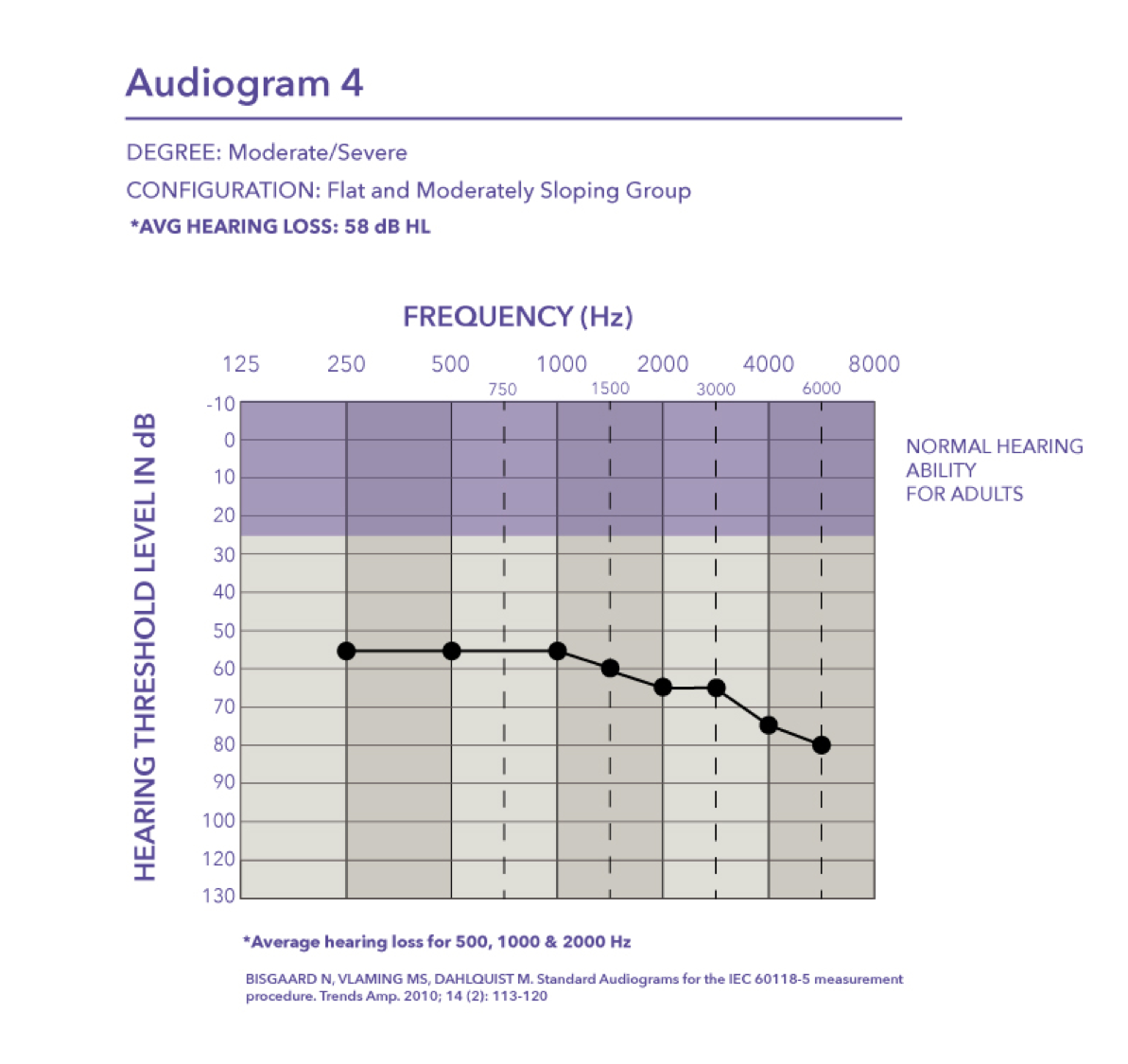
Severe. Audiogram five falls in the severe range with a pure tone average of 75 dB HL.
Very mild-precipitously sloping group. Audiogram six, very mild hearing loss with a pure tone average of 12 dB.
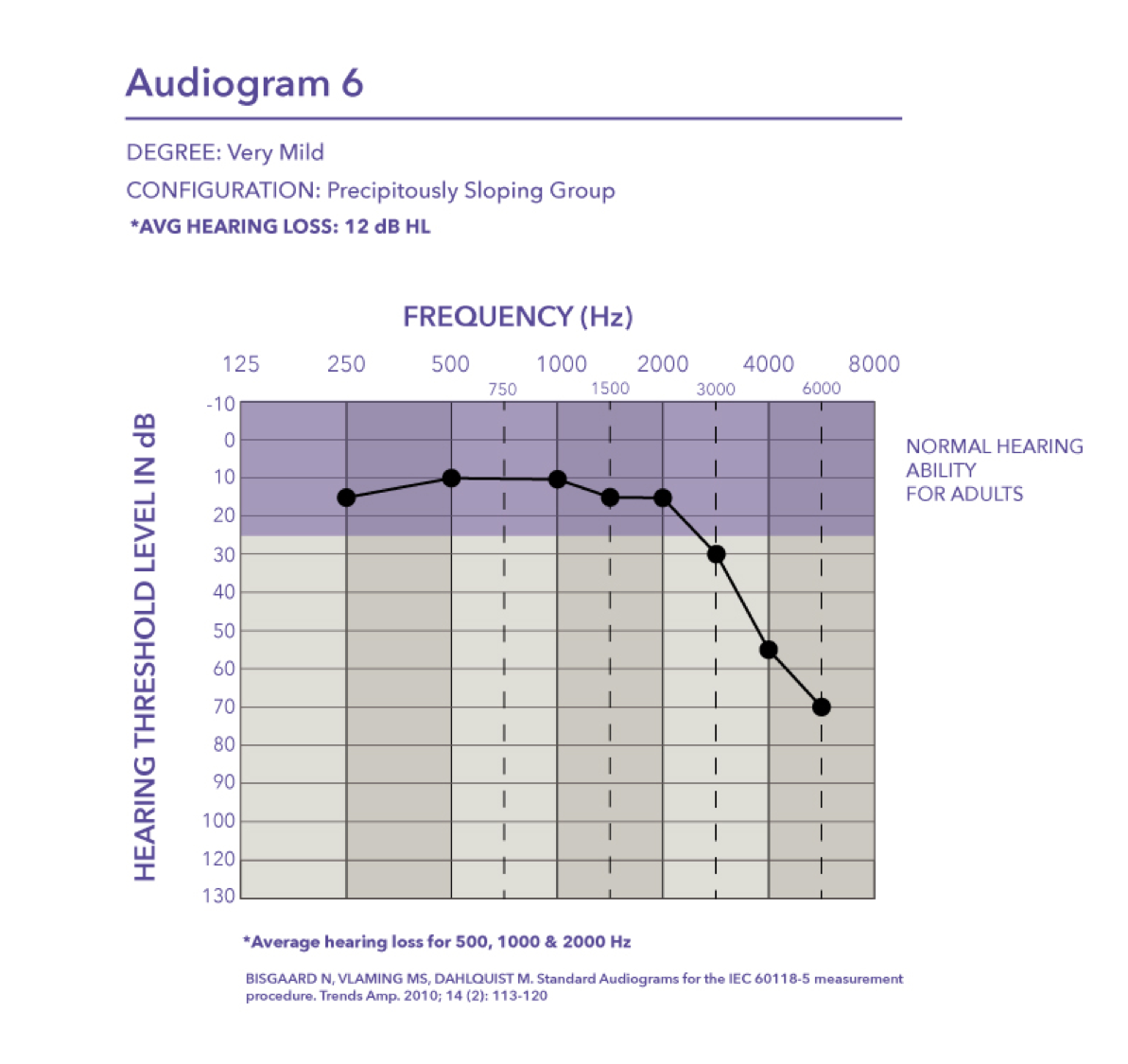
Mild-precipitously sloping group. Audiogram seven is a mild hearing loss with a pure tone average of 30 dB HL.
Moderate/Severe-precipitously sloping group. Then finally we have audiogram eight, which falls in the moderate to severe degree with a pure tone average of 57 dB HL.
Probe-Mic Measurements
These are the eight standard audiograms that we use during probe-mic measurements to determine the device's ability to match targets. We use two different intensity levels, soft and average. We take a look to see how well they match prescribed NAL-NL2 targets.
Target match definition. Now in this vetting process, NAL-NL2 targets are considered matched when the achieved output of a device is within plus or minus 5 dB, with the allowance for one target to be off more than a plus or minus five dB at a given intensity level. For each standard audiogram, two intensity levels (i.e., soft and average) and nine frequencies between 250 and 6,000 Hz are involved, which results in a total of 18 targets when adding the allowance to miss no more than one target per intensity. At a minimum, 16 of the 18 targets must be within plus or minus 5 dB for a device to be considered appropriate for that audiometric configuration. When you divide 16, 16 divided by 18, you end up getting 0.88. The quickest way to identify if a device has matched targets is to look for scores that are greater than 88%.
PSAP Qualifications Sold by Oaktree Products
In terms of PSAP qualifications that are sold by Oaktree Products, we vet PSAPs that meet the following criteria:
- Subjective impression of sound quality via listening check is deemed appropriate.
- Subjective assessment of functional performance is appropriate.
- OSPL90 tolerances are not greater than 120 dB SPL.
- Total harmonic distortion (THD) percentages are within acceptable tolerances.
- Equivalent input noise is no more than 28 dB,
- Average REAR for soft and average inputs match NAL-NL2 targets within plus minus five dB at least 88% of the time*
I put an asterisk there because early on, it used to be 83% at the time. As we started to get a little bit more confidence in making recommendations about PSAPs, we upped the ante to 88%. Now what you see here is an arbitrary key that was developed while we were working at Wash University initially on this PSAP project. It was designed to help us quickly assess whether a PSAP is a potential go, yield, or stop in terms of how it did on real ear measurements.
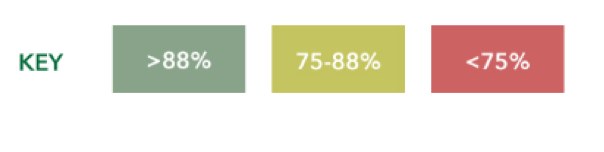
PSAPs scoring higher than 88% are going to be shaded in green because that to us is a potential go. This means they met NAL-NL2 targets for a particular standard audiogram and might provide the necessary output to generate meaningful benefit to the user. Those scores between 75 and 88% get a cautionary yellow. This means that they did not meet the minimum NAL-NL2 targets. Then any device scoring lower than 75% is basically in red because it's a stop meaning that this device did not match any of the NAL-NL2 targets or a sufficient amount. Therefore it's tough for us to believe that they are going to offer much real benefit to the user.
We also collect what we refer to as out of the box PSAP data (Objective assessment of baseline amplification with the device turned on and as packaged). Now this out of the box, PSAP data is not used to make decisions about whether or not a PSAP should be offered to our customer base or whether it's a potentially viable product. When we started looking at PSAPs and comparing them to hearing aid performance, we collected data on hearing instruments using first fit or manufacturer defaults, as well as verified fit, programmed fit using REAR. We wanted to mimic this approach with PSAPs. So out-of-the-box-testing of PSAPs represents the baseline amplification provided by a device when first turned on and using the dome that typically comes attached within the packaging. The reason we started doing this is we felt when a consumer opens it they are probably going to use the device as is, out of the box and simply turn it on. We just wanted to start gathering data from that perspective because we thought it might represent more of real-life use of a PSAP.
Performance Profiles
We are going to look at performance profiles of specific devices. I came across an article online, "The 18 Best PSAP Hearing Devices of 2018". It is available on everydayhearing.com, an entity that describes itself as "a leading provider of online hearing information with the commitment to bring the most credible and relevant hearing information online." From this 2018 article these are the top 10 of the 18 best PSAPs:
- LifeEar Boost
- Otofonix Elite
- PockeTalker Ultra
- Tweak Focus
- RCA Symphonix
- Digital Discovery
- Sound World Solution's CS50+
- PockeTalker 2.0
- Etymotic Bean
- Audicus SOLO
When I started preparing for this presentation later in the year, I referred back to this publication and couldn't find it. I realized there was an updated version that was now available which talks about "The 18 Best PSAP Hearing Devices of 2019". There have been some minor changes; Tweak was replaced by Nuheara IQBbuds Boost. Digital Discovery was replaced by the Bose Hearphones, not to be confused with the Bose over-the-counter hearing aid that we have heard about. Lastly, number 10 has been replaced with the ZVOX VoiceBUD.
In terms of which devices I wanted to take a look at, with the support of AudiologyOnline and Oaktree Products, I purchased a few products. I wanted to look at these first two. The LifeEar Boost and Otofonix Elite were of interest because they've been ranked the highest PSAPs two years in a row. I wanted to take a look at a couple of PSAPs that have been making repeat performances in different research studies: Sound World Solution's CS50 and the Etymotic Bean. I also wanted to examine the ZVOX VoiceBUD because it was an unsolicited demo that came across my desk, so I figured why not. Then I'm going to have a very quick comment about the Nuheara IQ Boost as well as the Bose Hearphones. To recap the PSAPs, we will discuss today are:
- LifeEar Boost
- Otofonix Elite
- Sound World Solution's CS50 (SWS CS50+)
- Etymotic Bean
- ZVOX VoiceBud
- Nuheara IQ Boost
- Bose Hearphones
LifeEar Empower BOOST
The LifeEar Empower Boost can be considered an intermediate level PSAP. This is because it offers the ability for the user to change volume as well as toggle through four different program settings. This PSAP was purchased from Amazon for $299. The Empower website offers the same product for about $399. At one point, this PSAP was selling for $549. It provides 12 bands of digital signal processing, utilizes digital noise reduction, output limiting, feedback cancellation, and WDRC. This device operates off of a size 10 hearing aid battery. If you want to turn it off, you simply have to open the battery door. The control switch adjusts volume as well as programs. It works in the same fashion as described with the PLAID when you're accessing the four programs. The first program available is referred to as an all frequency which provides amplification across the entire spectrum. Program two boosts the highs only. Program three is called All+NR, which is program one plus noise reduction. Then program four is program two with noise reduction. All the programs described in this manual are described as providing up to 40 dB of gain or amplification. This device comes with a 12-month warranty. Since this is sold on Amazon, I figured it would be interesting to see some of the customer reviews. Interestingly, 81% of these reviews by verified buyers are at least four-star. Comments range anywhere from "it works really well" to "it beats my $3,000 hearing aids", and "it's something that is very great for the price."
Findings. Figure 5 shows the electroacoustic analysis of the LifeEar Empower Boost using the protocol that I previously described. One need only look at these response curves to recognize that something isn't quite great with this product. For this particular readout, the Max OSPL90 was 100 dB at 4700 Hz. The equivalent input noise was recorded as 44 dB with an average full-on gain of minus seven dB. When you look at this information, it should come to no surprise that somebody out there bought it and said wow, the quality is poor.
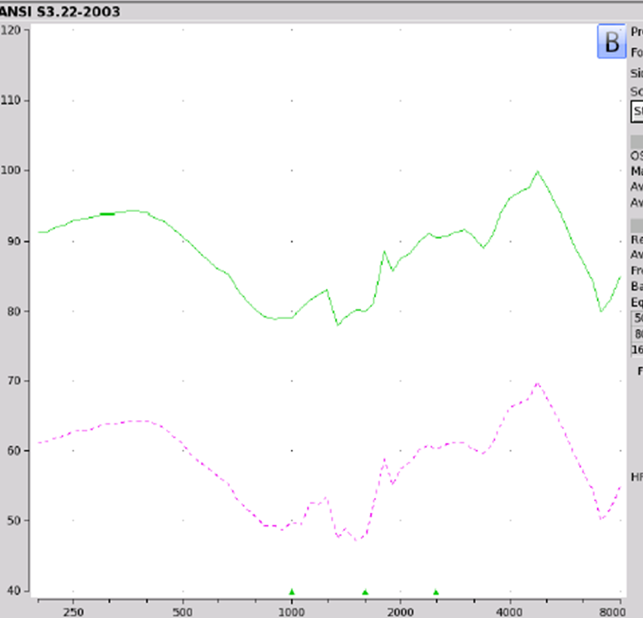
Figure 5. LifeEar Empower BOOST electroacoustic analysis.
Table 1 shows the percentage of NAL-NL2 targets the LifeEar Empower Boost met for the eight standard audiograms. Recall that anything in green is a go, anything in yellow is a cautionary and overly generous yield and anything in red that you see is a stop. What you see here is that the achieved output of this device did not meet targets for any audiogram. As indicated by the yellow, maybe this device could be appropriate for somebody that has an audiometric configuration that you see from audiogram one and seven.

Table 1. LifeEar Empower BOOST targets.
Product summary. Now in terms of the negative reviews, interestingly enough only 13% are two-star or less. In terms of a product summary, it's been ranked the number one PSAP for two years in a row by Everyday Hearing. At best, if you're just looking at on-ear fitting capabilities, it can potentially be okay for up to a very mild to mild hearing loss. However, if you look at the electroacoustic analysis, it negates any potential that this device may have. Now with full disclosure and transparency, when I first saw the electroacoustic analysis of this device, I'd never seen anything like it. This is the original analysis. Of course, I sent it out to another audiologist to rerun. When they retested this, something very different came up. The Max OSPL90 for the same precise device set up in the same exact fashion, had a Maximum OSPL90 output of 126 dB at 668 Hz. I asked my colleague to rerun a third time. The same device came back at 115 dB in terms of Max OSPL90 at 845 Hz. I'm going to be sending this device back again for them to rerun it. What I'm beginning to think with this device is that it's creating a level of intermittency that I've never seen before. I am confident that the protocol both centers have been using to do ANSI testing on these products is not coming into consideration in terms of affecting the results.
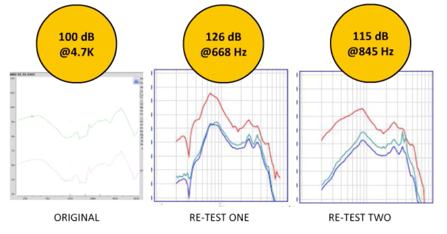
Figure 6. Comparison of the LifeEar Empower BOOST electroacoustic analysis.
Otofonix Elite
This is an intermediate level PSAP and works pretty much the same way as the previous one. I purchased from Amazon for $324; it works on a 312 battery. It comes with volume and program controls, offering four different programs from normal to noisy. All their literature indicates that it can provide up to 35 dB of amplification, and it comes with a 12-month warranty. In terms of verified purchases on Amazon, 81% are positive, ranging from "seriously awesome" and "excellent and amazing."
Findings. Figure 7 shows the electroacoustic analysis. The Max OSPL90 was recorded at 109 at 840 Hz. Equivalent input noise was 28 dB with the average full-on gain of 25 dB although their literature indicated 35 dB. When you take a look at on ear fitting capabilities, again, this device did not meet any of the NAL-NL2 targets. If you wanted to be overly generous, at best it might work for somebody who has an audiometric configuration as outlined in audiogram one. To refresh your memory, we are talking about adults who have thresholds of 10 dB threshold for air conduction through 2K Hz, 20 dB at 3K, 4K at 35, 6K at 40 dB. So it's highly unlikely that you're going to see anybody walking in your clinic with this type of audiometric configuration asking for amplification of any sorts. Even if they do, this one is a cautionary yield for only a very mild hearing loss. In terms of negative reviews, 11% are two stars or less.
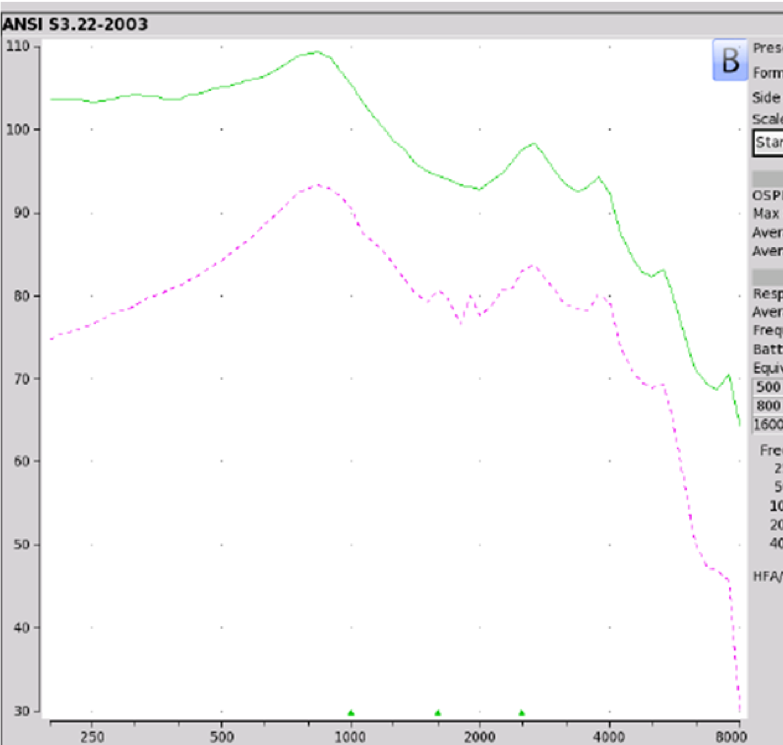
Figure 7. Otofonix Elite electroacoustic analysis.
Product summary. The Otofonix Elite has been ranked number two by Everyday Hearing now two years in a row. At very best, verified fit indicates that it might be potentially viable for a very mild hearing loss only. The gain is weaker than what is specified in all their marketing material.
Sound World Solutions CS50
This device is an advanced PSAP that works off of a smartphone, and you can use it to answer phone calls, to stream audio as well as music. It offers user customization for $349 and as a rechargeable device. It features three programs. One is for everyday life, the second one is for noisy situations, and the third one is for music. This device has been described as capable of meeting targets with the conclusion that it may be an inexpensive option for those with up to a moderate hearing loss. When we went ahead and evaluated the device using the vetting protocol previously described, we found that this device did not meet any of the NAL-NL2 targets for any of the standard audiograms.
Etymotic Bean
This is a very basic PSAP. It comes with a switch that gives you 15 or 23 dB of amplification and purchased for $214. In the past, it has been recognized as something that might be viable for somebody with a mild to moderate hearing loss. Unfortunately, we have vetted it twice, and we have found that it did not meet any of the NAL-NL2 targets. In terms of product summary for these two, the Sound World Solutions CS50+ and the Bean have been ranked in the top 10 in the past two years. Previous research has identified both products as appropriate for age-related hearing loss up to a moderate loss. The recent product vetting showed that NAL-NL2 targets could not be met for any of the standard audiometric configurations. The electroacoustic outputs for the Bean were weaker than what was specified in the manufacturer's specs.
ZVOX VoiceBUD (VB20)
This device was offered for me to review in consideration for our catalog. When I first looked into it, I was explicitly told that this was a PSAP. Interestingly enough, when I was following up on the product, I came across this device being advertised as a class one hearing aid. This confirmed suspicions that I've had in the past where some PSAPs are hearing aids that are repackaged in disguise. The long story short about the ZVOX VoiceBUD is that it did not meet any of the NAL-NL2 targets with the exception of the first, the audio one. Which again to refresh your memory is an audiogram with a PTA of 12 dB HL. So it's something that may potentially work in the event an individual has a very mild high-frequency hearing loss that starts at 4,000 Hz.
Nuheara IQBuds Boost
The Nuheara IQBuds Boost is a newer version of the original IQBuds and is a hearabble operated by a smartphone. It has the added feature that allows the user to customize the earbuds according to the results of a self-hearing test that is initiated by the device. So while I did not vet the IQBuds Boost, we did previously vet the Nuheara IQBuds. The original IQBuds which are available for $299 and the IQBuds Boost is available for $499. In vetting the original Nuheara IQBuds, these did not meet targets. I will say that this hearable performed better than most of the PSAPs that have come across my desk.
Bose Hearphones
The Bose Hearphones which are the conversation enhancing hearphones. They act just like any regular Bluetooth headphone while offering noise cancellation and the ability for the user to amplify speech. Bose Hearphones is a product that should not be confused with the future Bose hearing aid. The Bose hearing aid is going to be a self-fit device intended to amplify sounds for adults with perceived mild to moderate hearing loss. That is not available yet. Bose Hearphones retail for $499. It's not clear how much the Bose self-fitting over-the-counter hearing aid is going to resemble these Bose Hearphones. We'll have to wait and see because there are two different things. What I thought was extremely interesting is that the Bose Hearphones fared very well. They met NAL-NL2 targets for five of the eight standard audiograms and hit the yellow zone for two of the remaining three. So it'll be interesting to keep an eye on what Bose develops. A lot of the feedback that I get about the Bose Hearphones is interesting. People say it sounds great. The biggest complaint is occlusion. This is my personal bias; I can't stand the form factor. Some people can, and some people don't care. The critical takeaway is that this Bose Hearphone has been the first non-hearing aid device that I've seen in three years that has fared very well.
To recap on the product summary of the 2019 list, none of them are appropriate for an adult with mild to moderate hearing loss. The one exception may be the ZVOX VoiceBUD which tended to meet NAL-NL2 targets for only the very mildest hearing loss. So after vetting these, I wanted to get the feedback of some of my colleagues on which devices they were interested in examining. Responses include:
- Tweak
- PLAID
- CliKEZ
- BHA 220
- MD Hearing Aid Pro
Tweak, PLAID & CliKEZ
So the first three I'm going to be able to do all in one shot because these three devices are all from Ear Technology. They function the same way, and they mostly seem to have the same kind of performance potential. So just to refresh your memory, the CliKEZ is a hearing aid available through professional channels only. The PLAID is a PSAP available through professionals only but not on Amazon, versus the Tweak which is a direct-to-consumer PSAP that you're going to find on Amazon.
Findings. Figure 8 shows a reflection of the coupler measurements that were obtained on the Tweak, which were the same that we obtained on the PLAID and CliKEZ. They performed equally. The Max OSPL90 is about 110 at 800 Hz, equivalent input noise of 22 dB and the average full-on gain of 43 dB. Since the Tweak is sold on Amazon, there weren't a lot of customer reviews. When we take a look at ear fitting capabilities, the Tweak, as well as the CliKEZ, did not meet the majority of targets. The PLAID was able to meet NAL-NL2 targets for a very mild gently sloping hearing loss as well as for mild precipitously sloping hearing losses. The CliKEZ met targets for audio number six, which was also a mild precipitously sloping audiometric configuration.
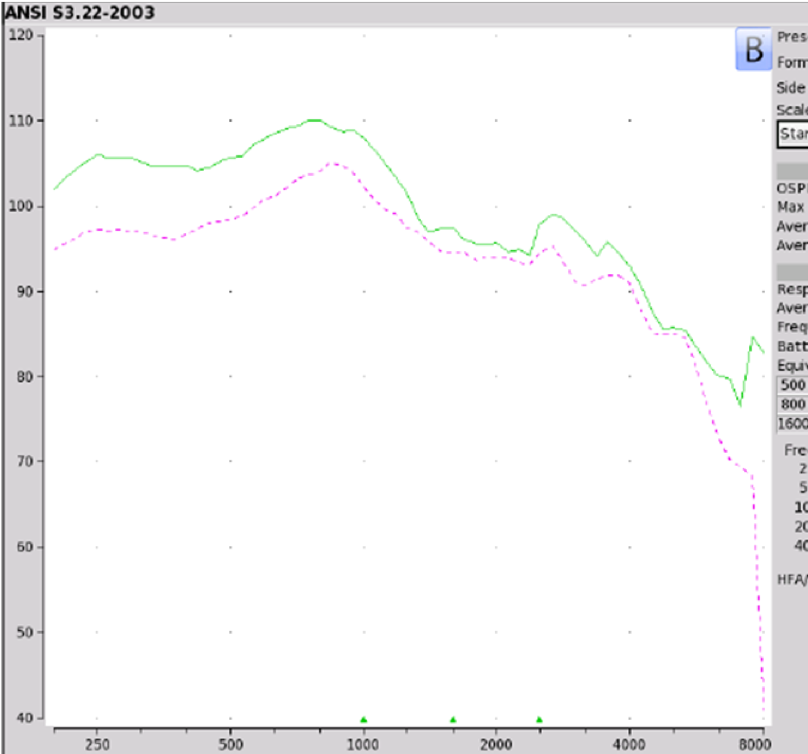
Figure 8. Tweak, PLAID & CliKEZ electroacoustic analysis.
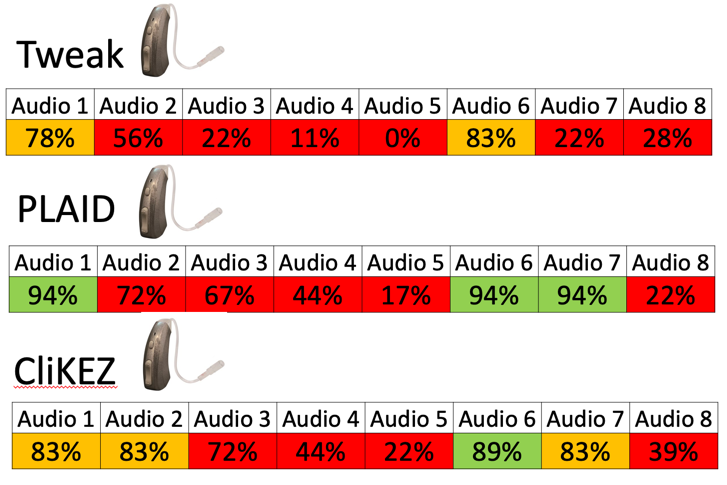
Table 2. Tweak, PLAID & CliKEZ targets.
Product summary. So to summarize, all products seem to be the same, just repackaged a little bit differently. The PLAID met NAL-NL2 targets up to a mild loss.
Britzgo BHA 220
This device is an intermediate level piece set that provides a volume control as well as the ability to change programs and was purchased on Amazon for $57. You can also find it available at Walmart and Walgreens and comes with a one-year warranty. There's an on/off switch, volume control, and program button. The device operates off of a 675 battery. In terms of the programs, you have one that is wide, which is across all frequencies, low-frequency, high-frequency emphasis, and speech emphasis. In terms of Amazon reviews by verified buyers, it's interesting to me that all of these PSAPs have an overwhelmingly positive customer review profile. 72% of Britzgo buyers on Amazon have at least a four-star rating.
Findings. Figure 9 shows the electroacoustic results. The Max OSPL90 of the Britzgo BH 220 is 123 dB at 1680 Hz. It has an equivalent input noise of 28 dB and an average falling gain of 33 dB. In terms of negative reviews, 17% have a two or one-star rating. If you look at the Max OSPL90, it's not surprising that this device has poor reviews.
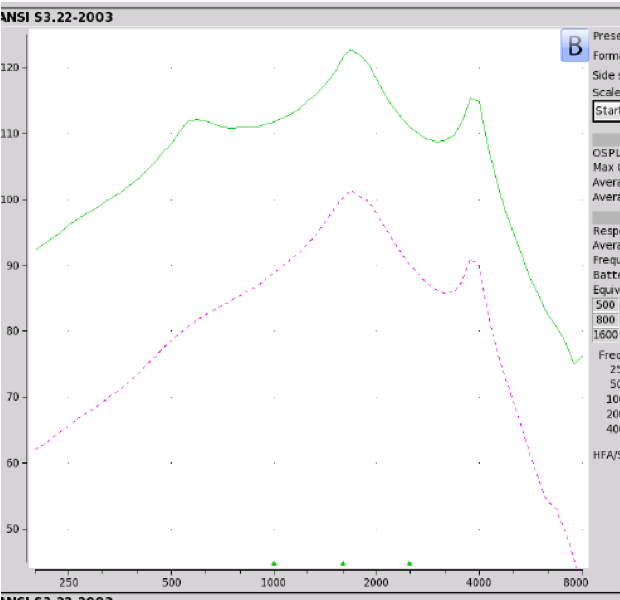
Figure 9. Britzgo BHA 220 electroacoustic analysis.
Product summary. This product did not meet any of the NAL-NL2 targets for the eight audiometric standard audiograms that we used. The Max OSPL90 exceeds what is deemed appropriate for individuals with mild to moderate hearing loss. This is a dangerous thing that is available out there for the consumer to purchase.
MD Hearing Aid Pro
The product is an analog hearing aid and not a PSAP. I purchased it at mdhearingaid.com for $199. The device comes with a volume control wheel and program switch. The program is either off, normal or high. It comes with a 90-day warranty. I would like to mention that after purchasing this product, I occasionally receive emails from "Sophia" at MD Hearing Aid Pro. The emails are reminding me to change my tubing and to do so at least every three months. There was a button for me to click if I wanted to reorder more directly from MD Hearing Aid Pro. By buying this hearing aid, I have an established relationship with customer service.
They will not allow you to return the device until 21 days from invoice because they feel that is how long you have to wear it to perceive any benefit. If you do decide to return it before this period you, will only receive 80% of your money back. If you receive a return authorization to ship the product back, you'll receive 90% of your money back, and after 45 days you don't receive any refund. I thought their strategy was interesting in terms of how they deal with returns. Regarding the four to five star reports, 93% are overwhelmingly positive.
Findings. If we take a look at the Max OSPL90 is 115 dB at 530 Hz shown in Figure 10. Equivalent input noise is at 19 dB. The average full-on gain again was 37 dB. If you take a look at on ear fitting capabilities, the MD Hearing Aid Pro did not meet any of the NAL-NL2 targets. At best if you feel overly generous, this device could be for somebody with a very mild hearing loss in the high frequencies.
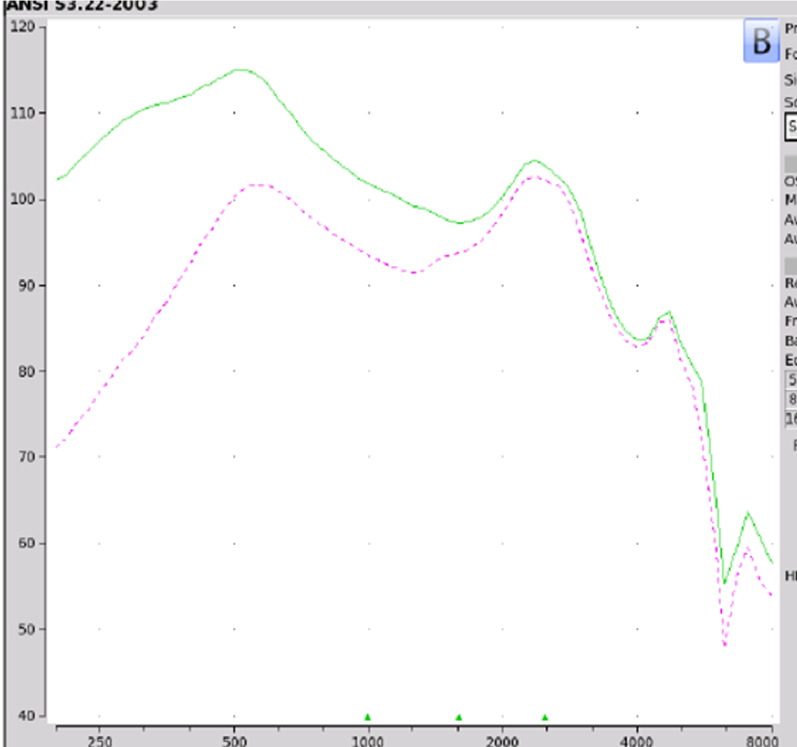
Figure 10. MD Hearing Aid Pro electroacoustic analysis.
The MSA 30X
This product is a basic PSAP that is available for $30 through Amazon and has a 30-day warranty. The device does have an on/off switch, volume control and is a rechargeable PSAP. I was interested in this device because the FTC felt that the company was deceptively advertising the MSA30X to consumers nationwide. They had claims that the product was independently tested which it was not, and that independent tests showed that the consumer could hear up to 30 times better. They were hit with a pretty hefty fine. Nonetheless, I was curious to see how this device faired.
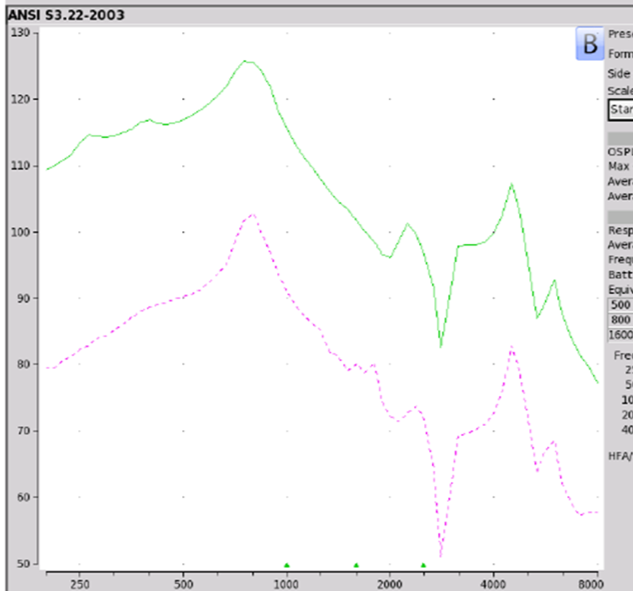
Findings. If we take a look at the electroacoustic analysis shown in Figure 11, the Max OSPL90 on this device is 126 dB SPL at 750 Hz, which is not a good thing. Equivalent input noise of 48 dB and an average full-on gain of 21 dB. The MSA30X, in terms of NAL-NL2 targets, did not meet any of them. When Reed, Betz, Lin, and Mamo (2017) took a look at this product to see how well individuals performed in unaided as well as aided conditions, what they found is that people who were fit with the MSA30X did poorer than when they were in the unaided condition. This is the one PSAP where there's information out there that shows that you're going to perform poorer. I will tell you a lot of people continue to buy this, probably because of the price point and the ridiculous marketing propaganda. Max 0SPL90 exceeds what seemed appropriate. It did not meet targets for any audiometric configuration, and it degrades speech understanding.
 .
.
Figure 11. MSA 30X electroacoustic analysis.
Distribution Models
I asked my colleagues, "would you offer PSAPs if you were the final decision-maker in your clinical practice?" Almost 60% indicated that yes they would, which led me to believe a lot of audiologists out there are thinking about it. 29.77% of clinicians indicated they would not offer PSAPs. I want to discuss PSAP distribution models. Every audiology practice will make their own decision as to whether or not to offer PSAPs as well as OTC. If so, how are you going to present it to your patients? After three years of vetting PSAPs and talking to colleagues, I've heard a lot of great points, both positive and negative. I'm going to sum it up with these three potential distribution models including some pros and cons as food for thought. The PSAP direct and PSAP indirect are models that integrate PSAPs within your clinical setting. Then there's a PSAP alternative, which I haven't come up with a very good name for but it's another distribution model that has directly resulted from PSAP infiltration.
PSAP Direct
This distribution model involves offering PSAP alternatives within your clinical practice without an intermediary. This can occur in one of two ways. You can set up a retail section within the reception area with off-the-shelf purchase options. So patients and consumers can freely purchase it as they wish. You can also offer your patients who decide they are not ready for hearing aids a PSAP. So when they walk out the door, they have something as opposed to nothing. This second scenario can play out in one of many ways. They can be directed to the retail section that is located in the waiting area, and independently choose an off-the-shelf product on their own. They can be designated to a dedicated area inside the clinic to self-explore two or three PSAP demos. They can try them out on their own and then decide on their own, as to whether or not they want to purchase it. Or you can recommend a specific PSAP that has demonstrated the ability to match NAL-NL2 targets for their audiometric configuration. You can have it in stock so they can buy it off the shelf. Your practice can do any combination of these, but this is how the PSAP direct distribution model could play out.
In terms of pros and cons, the advantages are you are offering a low-cost alternative which appears that most consumers are looking for. You can save time by directing to the area, and your staff can facilitate the off-the-shelf transaction. Some might say, although there are no data to support it, but perhaps it might be a gateway for the patient to return when ready for a hearing instrument. The cons include needing to keep inventory. Also, there's a need to vet the products and keeping up with the vetting process, which is something we're trying to do. There can also be some confusion from your patients. Some may say "wait a minute, you're the audiologist, you're supposed to help me, and now you're selling me this, but you're not doing anything with me." At the end of the day, the device may not work out and turn the user off. Keep in mind all the data that I've shown you on these PSAPs that met NAL-NL2 targets unless you fit and verify; there is no guarantee that those devices even if they are appropriate for their audiometric configuration are going to provide them benefit.
PSAP Indirect
I've heard a lot of audiologists say, "I want to offer it, but I'm a little bit concerned about drawing the professional line between this retail aspect and the professional component" or "I'm afraid that I'm going to create confusion." To combat this confusion, we have an indirect distribution model. This basically involves offering PSAP alternatives outside the boundary of your physical clinical office. One of the ways that I've seen this work pretty well is through an established shop that is set on your practice website. You might need a software add-on to your current website that is going to incorporate a shopping cart, as well as a merchant account. An individual can click and put things in a shopping cart, and the transaction all occurs online. Then you can turn around and send the device to the individual. That is how you can offer PSAPs indirectly to patients as well as the consumer.
In terms of pros and cons, the pros, it does draw a clear line in the sand because these transactions don't physically take place in your office. It can be looked at as an extension of your service and maybe a good way of branding your practice. There are ways to do this where you don't have to stock inventory. The cons, there are some startup and maintenance costs. You have to have access to IT, and there's a lot of marketing that is involved in getting this to work and at the end of the day. Once again for the reasons, I said before, the device may not work out and may turn off the user entirely from any form of amplification.
Hybrid Distribution Model
Finally, we have the third distribution model, where at one point, I started referring to it as the OTC hybrid model for lack of better terms. Unlike the other two models, the hybrid model does not involve offering PSAP options of any kind, directly or indirectly to patients or consumers. Instead, a patient will be offered a low-cost solution, since this is what they are asking for. It's not going to be in the form of a PSAP, but it's going to be offered under your terms and conditions that differ from a true OTC model.
Hearing aids have always been and currently, continue to be the gold standard for improving audibility for patients with hearing loss. Our research has shown that premium and basic hearing aids provide adequate amplification to meet NAL-NL2 targets for soft and average inputs for eight audiometric configurations. This is compared to PSAPs that as a group could only match for very mild or mild high-frequency hearing loss. Whether the outcomes of the OTC show that they are no different than PSAPs or maybe they are just as good as hearing aids, at the end of the day it doesn't matter. PSAPs and OTCs, even if it's decided that they do have a place with individuals with mild hearing loss, one needs to be knowledgeable of when and how to utilize such products. Audiologists are responsible for patient outcomes, yet the inherent nature of PSAP and OTC models exclude the professional component. This starts becoming somewhat of an ethical issue. There is no data to support the ability of a consumer to accurately match perceived level of hearing loss to their actual hearing. Thus without a current hearing test, a consumer coming into a clinic and asking to buy a PSAP can become problematic. Do you recommend a device that clearly does amplify areas of the audiogram, but you have no idea what their audiogram is? Even if you do have a current audiogram, and you recommend a device that seems to work for a particular audiometric configuration, you will not know the benefit unless you actually verify the fit. The whole design of OTC and PSAP is to bypass any of these professional services. There has been plenty of research to show that relying on manufacturer's first fit and not using real ear measurements have deviated from NAL-NL2 targets by as much as 10 to 20 dB particularly for the high frequencies (Hawkins & Cook, 2003).
Keeping this in mind, the OTC hybrid distribution model works something like this. You do not offer PSAPs. Instead, you make sure that you're providing a low-cost hearing aid. This basic hearing aid is dispensed using an unbundled model. The patient is educated in terms of what that unbundling means. This is the part that looks like an OTC delivery model. Unlike over-the-counter hearing aids, there's going to be one huge difference. The basic hearing aid is going to be programmed and fit, and the fitting is going to be verified one time only before they walk out that door. Because at the end of the day, audiologists are all about outcomes. A positive to this hybrid model is that it draws a clear line in the sand. You are leveraging your expertise, and you end up dispensing a quality hearing aid while verifying the fit, which is everything we should be doing. The cost may still be too high as a con, and it might just turn away consumers overall.
Summary
Hearing aids have outperformed PSAPs as a group. They are limited probably to a mild hearing loss and not moderate. Based on the results that we have been seeing, I questioned their benefit. There are some ethical considerations to keep in mind in the event you offer this option to your patients. As I wrap up, I know we covered a lot of information but do know that there is a PSAP database and a PSAP resource page available at oaktreeproducts.com. The PSAP resource is full of information. The database is full of updated data to show you coupler measurement and on ear fitting capabilities.
References
American National Standards Institute. Specifications of hearing aid characteristics. ANSI 53.22-1987. New York: ANSI.
Bisgaard N, Vlaming MS, Dahlouist M. Standard audiograms for the IEC 60118-15 measurement procedure. Trends in Amplification. 2010;14(2):113-120.
Bankaitis, A.U. (2017). PSAPs: Our role in this disruptive environment - regulatory issues & business practices. Audiology Online recorded webinar, course #30068.
Callaway S, Punch J (2008). An electroacoustic analysis of over the counter hearing aids. Am J Audiology 17(1):14 24.
Cheng C, McPherson B (2000). Ove -the•counter hearing aids: electroacoustic characteristics and possible target client groups. AudioI 39(2):110 116.
Chan Z, McPherson B (2015). Over-the-counter hearing aids: a lost decade for change. Biomed Res Int doi :10.1155/2015/827463
Consumer Technology Association (2017). Personal sound amplification performance criteria. ANSI/CTA 2051. Available https://standards.cta.tech/kwspub/published_docs/ANSI-CTA-2051-2017_FINAL.pdf
Hawkins, D. B., & Cook, J. A. (2003). Hearing aid software predictive gain values: How accurate are they?. The Hearing Journal, 56(7), 26-28.
Manchaiah V, Taylor B, Dockens A, Tran N, Lane K, Castle M, Gover V (2017). Applications of direct-to-consumer hearing devices for adults with hearing loss: a review. Clin Inter in Ag 12:859 8/1.
Reed N, Betz J, Lin F, Mamo S (2017). Pilot electroacoustic analyses of a sample direct-to-consumer amplification products. Otology and Neurotology 38(6):804.808.
Smith C, Wilber LA, Cavitt K. PSAPs vs hearing aids: An electroacoustic analysis of performance and fitting capabilities. Hearing Review. 2016;23(7):18.
Voss, Oeding, Bankaitis, Pumford & Valente (2017) Comparing Coupler, Real Ear, and Calculated 511 Between Selected PSAPs and Hearing Aids. A Guide For Audiologists.
Xu J, Johnson J, Cox R. Breitbart D (2015, March). Laboratory comparison of PSAPs and hearing aids. Paper presented at the annual meeting of the American Auditory Society, Scottsdale, AZ.
Citation
Bankaitis, A.U. (2019). Through the PSAP looking glass. AudiologyOnline, Article 24864. Retrieved from https://www.audiologyonline.com


