Editor’s Note: This text course is an edited transcript of a live seminar. Download supplemental course materials.
Today I will be presenting an overview of the Esteem® middle ear hearing implant. First, I want to consider the question, “Why aren’t more people with hearing loss using hearing aids?” In spite of a growing prevalence of hearing loss and significant advances in hearing aid technology, we continue to see a low uptake of hearing aids by the hearing-impaired population. At the University of Queensland in Australia, Louise Hickson and colleagues (2014) have identified three major facilitators of help seeking and successful hearing aid use. They include the following: positive attitudinal beliefs about hearing aids, support of significant others, and self-efficacy for hearing aids. I’d like to focus specifically on two: positive attitudinal beliefs and self-efficacy for hearing aids.
Why Aren’t More People Using Hearing Aids?
“Attitudinal beliefs” about hearing aids can sometimes be positive, such as the belief that hearing aids will help, and they can be negative, such as the perceived stigma related to hearing impairment, particularly in association with aging in our population. “Self-efficacy for hearing aids” refers to confidence in one's ability to insert, remove, and manipulate hearing aids, replace batteries, et cetera, on one's own. These two factors suggest that there is a place for totally implantable hearing technology; the Esteem hearing implant represents another option on the continuum of care for hearing-impaired individuals. It is an option for those seeking an alternative to traditional hearing aids.
Before I get into describing the Esteem technology, I want to focus first on lifestyle issues, as they are important to consider with regard to implantable technology. I want to let you hear some comments from Esteem recipients.

The Esteem is the only FDA approved, totally implanted, active middle-ear implant. It received FDA approval on March 17, 2010. The Esteem is appropriate for individuals with moderate to severe sensorineural hearing loss. The Esteem does not interface directly with the outer ear; it uses no speaker or artificial microphone, no external components, and requires nothing in the ear canal. In the diagram shown in Figure 1, you can see how the Esteem’s components are positioned in relation to one another. There are two transducers, a Sensor and a Driver, that are placed in the middle ear, and a Sound Processor that is implanted beneath the skin behind the ear. The Esteem is invisible. It can be used 24 hours a day, 7 days a week. It enables a person to swim, shower, or exercise without worry of damage or discomfort. It is waterproof and is safe for diving down to a depth of 33 feet. The Esteem requires no daily maintenance.
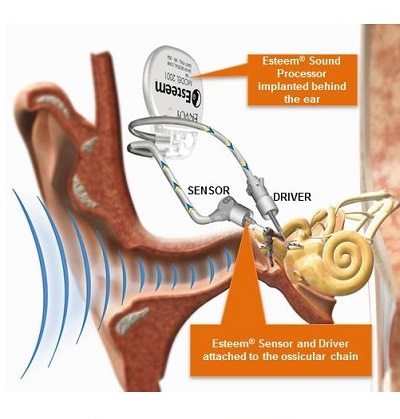
Figure 1. Components of the Esteem implant.
I am going to play a short animation, which can be found on our website. It illustrates in a simplistic way how the Esteem works, and it will give you some context as I talk about device function.
What makes the Esteem system unique is the way in which it uses the tympanic membrane (TM) as a microphone. This results in less required processing of input sound. The vibrations generated at the TM produce input signals naturally. It allows for retention of pinna and ear canal resonances, which result in spectral shaping of acoustic sound waves as they approach the TM, producing a net reduction in the amplitude of sounds below 1000 Hz and a net increase in the amplitude of the spectrum above 1000 Hz. You can see in Figure 2 that the combined transfer function of canal and pinna resonances give us a resonant peak in the region of 2 to 5 kHz.
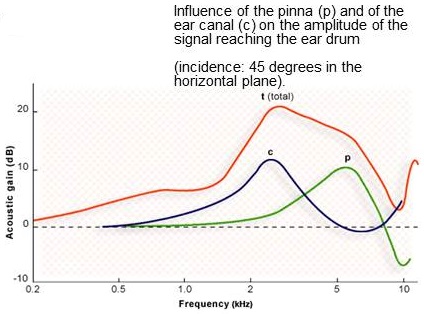
Figure 2. Combined transfer function of canal and pinnae resonances. From Journey into the World of Hearing (www.cochlea.eu) by Remy Pujol et al., NeurOreille, Montpellier. Used with permission.
The Esteem System
The Esteem is an amplification system, and it can be thought of as analogous in some ways to conventional hearing aids, even though it is entirely implanted. The Sensor is a transducer that picks up vibrations from the incus as the input signal, comparable to the microphone in a conventional hearing aid. The Driver delivers an amplified output signal as mechanical energy to the stapes, comparable to the receiver or speaker in a conventional hearing aid. The Sound Processor processes and amplifies acoustic signals according to programmed settings and algorithms. This is comparable to the processor in conventional hearing aids.
Components
There are three fully implanted components in the Esteem system: a Sensor, which is situated at the front end of the system; the Sound Processor, which is where signals travel from the Sensor to be shaped and processed; and the Driver, which is at the end of the system near the cochlea where the signal is delivered to the inner ear. These three components work in series to provide amplification of the input signal propagated through the TM, malleus, and incus.
The Esteem uses custom integrated circuitry with low energy requirements. The frequency range (in electrical terms) is 200 to 6000 Hz, with up to 50 dB of available electrical gain. A non-magnetic, biocompatible Sound Processor case houses the battery and the processor. The battery takes up most of the space in the Sound Processor case. The lithium iodine battery lasts four-and-a-half to nine years. The median battery life is about five-and-a-half years. Battery life will depend somewhat on input sound levels and on the amount of gain that is programmed into the Esteem.
Broadband filters absorb electromagnetic interference, preventing most electrical interference. Inductive bidirectional telemetry is used to interrogate and program the implanted Sound Processor. Interrogation refers to reading the settings that are currently programmed in the sound processor, and programming allows the audiologist to manipulate the settings programmed into the sound processor. This programmability allows the audiologist to customize a fitting for each patient's needs.
Sound Processing
The Esteem uses analog signal processing, but with digital control. This can be thought of as a “hybrid” processing technique. The reason behind the analog processing relates to power consumption; analog signal processing requires little power, compared to digital, and is how the system was designed in order to achieve a multiyear battery life. In addition, analog processing is a natural way of processing the input sound captured at the TM. Digital control, however, allows for greater programmability of the analog signal processor.
Piezoelectric Transducers
The key to the Esteem's operation lies in the use of piezoelectric transducers. The Sensor and Driver are both piezoelectric transducers. They are composed of a crystal aggregate material, sometimes referred to as a bending bimorph. Layers of materials such as lead and titanium oxides are sandwiched together, structured in such a way that they can move only in one dimension -- similar to a diving board that can move back and forth in only one direction. This directionality allows for reversible electromechanical transduction. When a force is applied, or when this piezoelectric transducer is deflected in one direction, a voltage is generated. Conversely, when a voltage is applied to the piezoelectric transducer, motion or mechanical deflection is generated.
The Esteem Sensor and Driver are piezoelectric transducers. The Sensor is activated when displacement of the incus (by acoustic energy at the TM) deflects the attached Sensor, which then produces an electrical signal that is sent to the Sound Processor. At the Driver, the electrical signal from the Sound Processor causes the reverse to occur -- deflection of the Driver, and this vibrational motion is transferred to the stapes. These transducers use very little power. They produce low distortion, even at maximum output levels, and very low noise. They are both housed in titanium enclosures that are 100% hermetically sealed for safe, reliable, long-term implantation.
Sound Processor
The Sound Processor, implanted behind the ear, is situated between the Sensor and the Driver in the pathway of sound. It receives an electrical signal from the Sensor and shapes the signal in terms of frequency and gain and then sends the modified signal to the Driver, attached to the stapes. The current generation Sound Processor is a two-channel, analog processor. The Sound Processor is easily replaced and is backward-compatible, allowing for future technology upgrades. It can store three programmable listening profiles. It utilizes output-controlled compression, and it is 100% hermetically sealed for safety and reliability.
Sensor
In the middle ear, the Sensor abuts the body of the incus bone so that as the incus vibrates, the piezoelectric element within the Sensor flexes, creating an electrical signal that travels to the Sound Processor. The Sensor is connected to the Sound Processor by a detachable lead that can be easily disconnected right at the Sound Processor, allowing for battery replacement by simply unplugging that lead’s attachment at the Sound Processor, swapping in a new Sound Processor, and then plugging the Sensor lead back in. The Sensor is sensitive, energy-efficient and linear over a wide dynamic range.
The Driver, at the other end of the system, is connected to the stapes. It receives electrical signals from the Sound Processor, causing the piezoelectric element within it to flex, causing vibration of the stapes. These vibrations are then delivered through the stapes to the cochlea, in the normal fashion. It is housed in a hermetic titanium enclosure for reliable long-term implantation, and like the Sensor, is connected to the Sound Processor by a detachable lead.
Software
The Esteem Commander is the name given to the software that is used to evaluate device performance and program the Sound Processor. This fitting software is accessed currently through a dedicated laptop computer provided by Envoy Medical, and it communicates with the implanted device via a telemetry wand, which is simply the Personal Programmer (the remote control that the patient uses to manipulate volume steps and profile settings) connected to the computer with a USB cable. The function of the Commander is to interrogate and program the implanted Sound Processor, and to perform some diagnostic tests for assessing device performance.
These device diagnostic tests include the Envoygram, a test that assesses Driver performance in isolation from the Sensor. The device is set to very low gain, and electrical signal is delivered directly to the Driver. The Sensor is not active. Behavioral thresholds in response to the signals to the Driver are measured, and the level of electrical stimulation to the driver producing a detectable sound for the individual is recorded.
The Sensor test evaluates Sensor performance in isolation from the Driver. Sound is delivered to the ear of the Esteem patient via an insert earphone, and the voltage produced at the Sensor is read by the software.
A Feedback test is used to determine if the potential for mechanical feedback is outside of acceptable limits for the Esteem. The Esteem does not produce acoustic feedback in the same way that hearing aids do, but any closed system with input and output signals is capable of generating some kind of feedback. In the case of the Esteem, it is mechanical or vibrational feedback, which will occur when the Sensor is able to detect a signal delivered at the Driver. Normally, these two transducers are isolated enough from one another that we can achieve sufficient levels of gain without a feedback loop being created. An additional test, the Max Gain test, uses an automated algorithm to determine the maximum amount of gain that the Esteem can provide at fixed parameter settings, without generating feedback.
The Personal Programmer is the remote control that is used by the patient to adjust volume and to select one of three profiles stored in the Sound Processor. There are various buttons, all requiring pressure from the finger, to activate a query or to change a profile or a volume step. There are also battery lights, one related to the battery within the Personal Programmer, which uses AAA batteries, and the other relating to the Esteem Sound Processor battery. This will illuminate when the battery is close to depletion and it is time to replace the Esteem Sound Processor.
Fitting the Esteem
Prescriptive gain targets can provide guidance in fitting any kind of amplification, and the Esteem is no exception. We use prescriptive gain targets with the Esteem to try to determine the amounts of gain that an individual needs. However, there is a bit of a difference. With hearing aids, we can determine the desired amount of gain as a function of frequency for a given hearing loss, and then we can verify if we have achieved those gain targets by measuring insertion gain with real ear measures. With the Esteem, however, we cannot measure insertion gain or, in this case, actual output at the stapes footplate. We are limited to using behavioral methods, or functional gain thresholds.
The final frequency response of the Esteem, after we have programmed in all the electrical gain and frequency response characteristics that we want, is going to be dependent on individual anatomy and transducer placement, in addition to the gain and frequency response that we have tried to shape through our programming. Consequently, verification of a fitting is limited to behavioral threshold measures or functional gain. Another way of saying this is that the same set of parameter settings and gains can result in different amounts of functional gain for one individual versus another, due to differences in anatomy, transducer placement, and individual differences among patients.
The fitting parameters that we do have control over are shown in Figure 3. There are three primary fitting parameters for each of the two bands: cuts, crossovers, and gains. We can adjust the low-cut frequency and high-cut frequency. These will determine the skirts of the transfer function of the system.
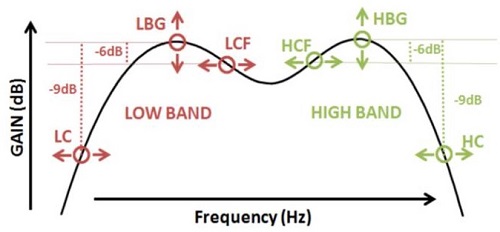
Figure 3. Fitting parameters.
In addition to that, we have control over the crossover frequencies, which will determine how close the two bands are to each other in the frequency domain. If we set the crossover frequencies very closely, then we will get an additive effect between the two bands, resulting in a greater mid-frequency resonant peak. Our current fitting strategy calls for separating those crossover frequencies as much as possible, to try to avoid that resonant peak in the middle of the frequency range. Lastly, we have band gains for both the low and high bands.
The closest thing we have to electroacoustic specs for the Esteem is actually based on behavioral measures in a large group, several hundreds of patients. If we look at the effect of the high-band gain parameter, based on average performance across patients, we can see in Figure 4 that the 5 dB programmable options for high-band gain will result in approximately 5 dB changes in thresholds for most of the low band, across patients (in these illustrations, discrete thresholds have been connected with smooth curves). The same goes for the low-band gain parameter, which is also programmable in 5 dB increments from 5 to 40 dB. For each of the different gain steps, we see a pattern of performance that reflects that 5 dB difference in gain. The low-cut parameter will affect the steepness of the skirt on the low-frequency end of the frequency response, and the high-cut parameter is going to have the same effect at the high-frequency end of the response.
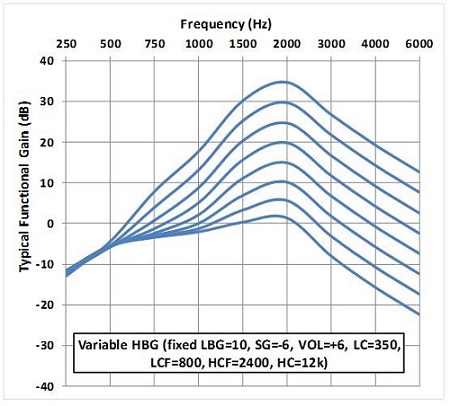
Figure 4. High-band gain parameter (HBG).
Volume will affect both bands equally. There are 16 different possible volume steps; however, we usually constrain the minimum and maximum volume to be much less than those 16 possible steps, which is more than the typical patient needs. Each volume step is 3 dB, and we have determined that system performance is optimal at settings of -6 dB to +9 dB.
The Esteem also has output-limiting compression that is programmable. The maximum power output (MPO) can be set to 100, 97, 94, or 88 dB SPL. Those are not very high MPOs, and we generally recommend the maximum 100 dB SPL setting for MPO, to avoid saturation of speech at high levels. The attack and release times for the output compression are 6 msec and 5 msec, respectively. We do not have wide dynamic range compression (WDRC) available in the current Sound Processor, but we anticipate that it will be available in a future generation processor. (Input compression was designed into the system several years ago, but the time constants were such that it was not very well accepted by patients, and so it was removed).
Clinical Outcomes
I would like to talk briefly about our pivotal clinical trial outcomes. We conducted an Investigational Device Exemption (IDE) pivotal clinical trial, which led to FDA approval, beginning in January 2008. That trial involved three surgical sites with 57 patients. The primary outcome measures included speech reception threshold (SRT), word recognition at 50 dB HL, the incidence rate for serious adverse device events, and residual cochlear function measured by bone conduction and by the EnvoyGram test that I mentioned earlier.
Secondary endpoints included the Abbreviated Profile of Hearing Aid Benefit (APHAB) and an Esteem-specific quality-of-life questionnaire. The results from the clinical trial for SRT are shown in Figure 5. Along the x-axis are the time points. The red bar is the unaided baseline condition. The average preoperative unaided SRT across all 57 patients was about 58 dB. Then, you see the measurements for 4-month, 10-month, 2-year, 3-year, and 4-year time points. All the thresholds are right around 30 dB. We are seeing an improvement in SRT very close to 30 dB. We do have some data for the 5-year point, as well, but it is not complete yet.
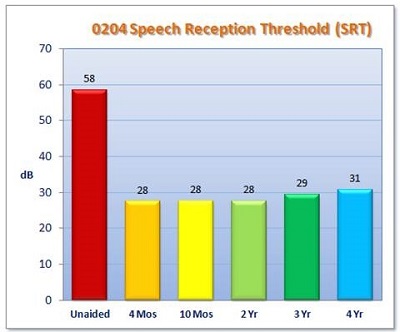
Figure 5. IDE clinical trial results for SRT. Measurements were taken at 1 month, 4 months, 10 months, 2 years, 3 years, and 4 years.
The results for word recognition measured at 50 dB HL are shown in Figure 6. Again, we are comparing to unaided (red bar), which was poor at 10%. That is reflecting the poor audibility of speech at that presentation level for most of the subjects in that cohort. Word recognition rose to about 75% correct, postoperatively. This is for monosyllabic words, tested at 4-months through 4-years.
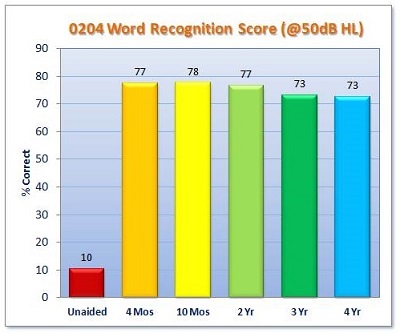
Figure 6. IDE clinical trial results for word recognition scores at 50 dB HL.
The next graph (Figure 7) shows the mean audiometric results at the 10-month data collection point. The blue line indicates baseline hearing function. Functional gain measures with the hearing aid are the red symbols, the best functional gain obtained with Esteem are the green symbols. We are seeing something that correlates fairly well with what we saw for SRT in the previous slide.
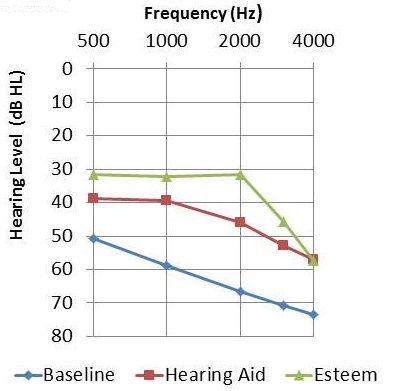
Figure 7. Mean audiometric thresholds for baseline unaided (blue), hearing aid functional gain (red), and Esteem functional gain (green).
The pure-tone average (PTA) seems to be stable over time (Figure 8). The PTA (average of 500, 1000, and 2000 Hz) in the unaided condition is represented by the red bar. For each of the measuring points, we see that it mirrors the SRT results closely, and has held stable for four years.
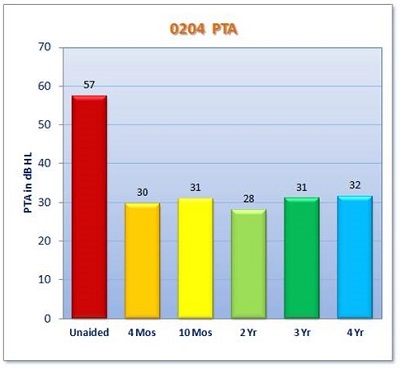
Figure 8. Average PTA across all clinical trial subjects over four years.
Since the clinical trial, we have been collecting data in a not-so-controlled way, but enough that we can compare the performance of post-approval commercial patients to the clinical trial patients (Figure 9). Here you see IDE performance, which refers to the pre-market approval clinical trial results, and USA performance, which refers to post-approval commercial patients in the United States. You can see that the average audiogram is similar across both those groups, suggesting that we're seeing the same outcomes in the commercial patients as we did in the clinical trial group.
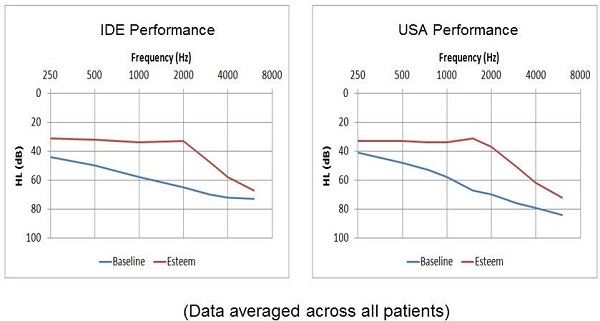
Figure 9. Comparison of audiometric results between subjects in the IDE clinical trial and post-approval commercial patients.
The APHAB was administered to the clinical trial group (Figure 10). The blue symbols represent the baseline unaided responses to each of five subsections of the APHAB: global, ease of communication, background noise, reverberation, and aversiveness. The red symbols refer to baseline HA-aided results. All of the clinical trial patients used and were appropriately fit with a hearing aid prior to being implanted with the Esteem. Results with the Esteem are indicated by the green symbols. This graph indicates greater perceived benefit from the Esteem than from the preoperative hearing aid.
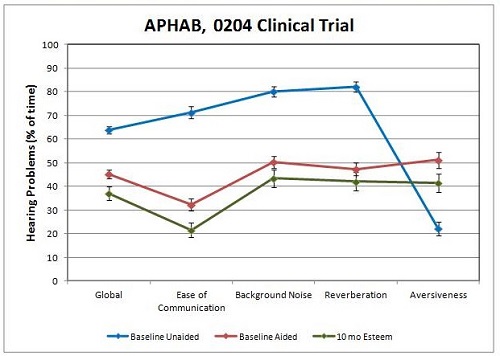
Figure 10. Mean responses to the APHAB across all trial subjects. Baseline unaided (blue), baseline aided (red), and 10-month post-operative with Esteem (green).
There was also an Envoy-specific quality-of-life questionnaire that was administered to the trial patients. The questions generally related to activity level, lifestyle issues, and hearing, comparing the Esteem to the person’s pre-implant hearing aid. Eighty-six percent of subjects reported that their activity level was somewhat or much better with the Esteem (Figure 11). That would be expected, because the Esteem offers greater freedom to be active than do hearing aids. Sixty-seven percent of subjects reported understanding conversation when several people are talking as somewhat or much better with the Esteem.
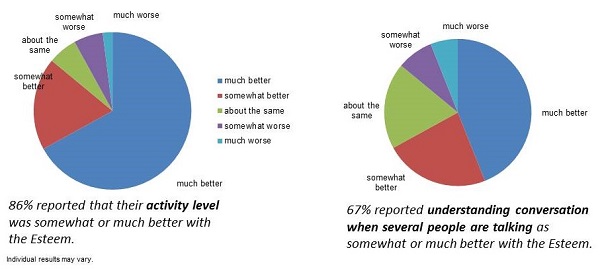
Figure 11. Results from the Esteem quality-of-life questionnaire.
We did have a speech-in-noise measure in the clinical trial, the QuickSIN. There was no significant difference between preoperative hearing-aided and postoperative Esteem-aided performance. We did not show an objective advantage of the Esteem in background noise, but we did get subjective reports that patients felt they did better in noise with the Esteem. On the quality-of-life questionnaire, seventy-eight percent of respondents rated clarity of sound as somewhat or much better with the Esteem. Seventy-one percent of respondents rated the ability to understand speech in background noise as somewhat or much better with the Esteem. Eighty-one percent said their feeling of confidence was somewhat or much better with the Esteem, and 77% reported voices sounding natural as somewhat or much better with the Esteem. Interestingly, 69% rated the benefit of the entire system being invisible to the onlooker as somewhat or much better with the Esteem. One might expect that percentage to have been higher. It possibly suggests that invisibility was not a primary driver for these individuals seeking the Esteem implant.
Surgical Considerations
The Esteem consists of small components that are situated in the middle ear space. Obviously, there needs to be enough space in the middle ear to accommodate these transducers. Prior to final determination of candidacy, CT scans are obtained to ensure that the patient's anatomy is compatible with the Esteem. Figure 12 shows a schematic of the transducers that has been overlaid on a CT scan, to illustrate the importance of the physical space requirements.
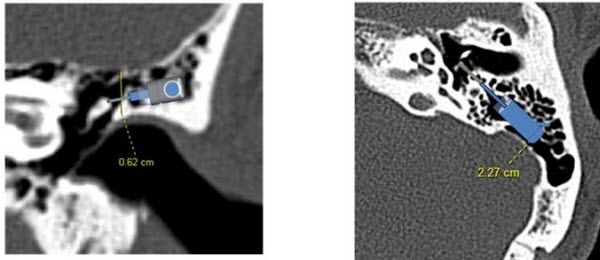
Figure 12. Overlay of Esteem transducer components on a CT scan to show how the parts will be accommodated within the middle ear space.
Surgical Steps
This first surgical step is creating the sound processor bed. Similar to a cochlear implant receiver stimulator, it is going to require drilling a small bed in the skull behind the ear so that the device can sit down flat against the head. Next, a mastoidectomy and atticotomy are completed, and the facial recess is drilled out. This surgery requires drilling to the limits of the anatomy of the middle ear, in order to create enough space for the system. At this point in the surgery, intact chain displacement measurements are taken. This involves laser Doppler imaging, performed by placing small markers on the ossicles, presenting sound into the ear, and measuring the movement of the ossicles in response to sound. It is important that the ossicles are moving normally, because the system depends on ossicular movement in order to function optimally.
Once those intact chain displacement measurements are taken and determined to be within our specifications, the long process of the incus is resected. The ossicular chain has to be disarticulated in order for the system to work, for the simple reason that feedback can occur very readily if the chain is left intact. The incus is resected, and the stapes is then prepared. At this point, the Sensor and Driver transducers are put into place next to the incus and the stapes. They are attached with special cements. Once everything is in place, before closing, intraoperative testing is performed to ensure that everything is working normally. Each of the transducers in isolation is tested, followed by the system as a whole.
After approximately five to six years of use, the Esteem’s battery will become depleted. At that point, the Sound Processor, containing the battery, needs to be changed out. This procedure can usually be performed under local anesthesia as an in-office procedure. It takes about 60 minutes. The new Sound Processor is initially set to factory specifications, at very low gain settings, before it is connected to the patient’s implanted Sensor and Driver leads. The patient, who is awake during this procedure, will verify that confirmation tones from the Personal Programmer are audible with the new Sound Processor, and then the incision is closed. The patient will be sent to the audiologist for device programming.
Explant and Ossicular Reconstruction
Infrequently, a device needs to be explanted. Remember that we disrupted the ossicular chain during surgery; it will need to be reconstructed if the device is explanted. Common methods of reconstruction are the K-Helix, Envoycem bridge and the PORP/TORP (partial ossicular replacement prosthesis; total ossicular replacement prosthesis). The K-Helix (Figure 13) was developed by one of our Esteem surgeons, Dr. Eric Kraus, specifically for post-Esteem reconstructions. Sometimes a cement bridge will be used instead; Envoycem, a type of cement used in the surgery, can be used to bridge the gap in the incus. Alternately, a more standard PORP/TORP procedure can be used.

Figure 13. K-Helix reconstruction device.
Candidacy
Who is a candidate for the Esteem? The FDA indications are as follows. The patient must be 18 years of age or older with stable, bilateral, moderate to severe sensorineural hearing loss, defined strictly as a PTA of 40 to 90 dB HL. Unaided word recognition must be better than or equal to 40% correct on monosyllabic words. Candidates must have a normal-functioning Eustachian tube, a normal TM and middle ear anatomy. Adequate space is needed for the Esteem implant, determined by high-resolution CT scan. Candidates need a minimum of 30-days’ experience with appropriately fit hearing aids.
Even though we cite FDA indications of 40 to 90 dB PTA, Envoy recommends constraining that fitting range even further.
In Figure 14 (note that 20 dB is at the top of the y-axis range), the orange shaded region indicates the range of audiometric thresholds that are most likely to achieve prescriptive gain targets with the Esteem, with sufficient dynamic range to enable good speech recognition. The upper limit of this range is determined in part by system noise. Any type of electronic system will have circuit noise, and the Esteem is no different. For patients who have normal hearing in the low frequencies, with thresholds of 10 to 20 dB HL, there is a good possibility that they are going to hear low-frequency system noise. For that reason, steeply sloping hearing losses with normal thresholds at 250 and 500 Hz are not ideal candidates, audiometrically, for the Esteem.
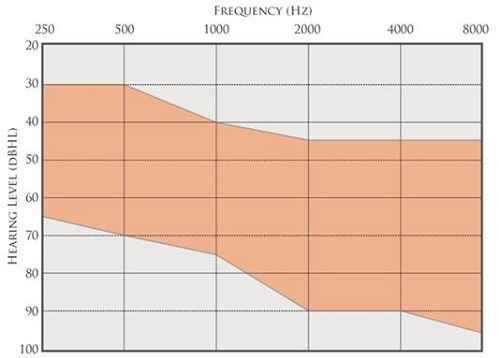
Figure 14. Ideal fitting range for Esteem. The orange shaded region is the range of audiometric thresholds that are most likely to achieve prescriptive gain targets, with sufficient dynamic range to enable good speech recognition.
The lower limit of this ideal fitting range (Figure 14) is dictated in part by the maximum output of the system. The MPO is about 100 dB SPL, so if hearing losses extend up into the range of 80 to 90 dB HL, especially in the low frequencies, they are going to have a very limited dynamic range available to them before sound is pushed into saturation, producing distortion. We have designed the lower limits of the ideal fitting range to allow for a reasonable dynamic range and for a reasonably good predicted fit to prescriptive gain targets. Again, the 40% word recognition requirement is a very important consideration. A candidate with >40% WRS is considered off-label; if you have a patient with scores poorer than 40%, you might need to consider the possibility that the patient is a cochlear implant candidate.
Contraindications
Contraindications for the Esteem include a history of post-adolescent chronic middle ear infections, inner ear disorders, recurring vertigo requiring treatment, disorders such as mastoiditis, endolymphatic hydrops or Meniere’s disease, known history of fluctuating conductive and/or sensorineural hearing loss over the past one year period (this is defined very specifically as 15 dB in either direction at two or more frequencies from 500 to 4000 Hz), an air-bone gap indicating a conductive or mixed hearing loss, history of otitis externa or eczema of the outer ear canal. This seems almost counterintuitive, because you might think that a patient like this would have difficulty wearing traditional hearing aids and might be an excellent candidate for the Esteem. I think this contraindication is related to the possibility of infection of the incision site. There may be a propensity for infection in general.
Others contraindications include cholesteatoma or destructive middle ear disease, retrocochlear or central auditory disorders, and disabling tinnitus, defined as tinnitus that requires treatment. As with traditional hearing aids, we often hear reports of patients who find the annoyance of their tinnitus is alleviated by the Esteem, particularly since the Esteem can be used at night, unlike hearing aids.
Another contraindication is history of keloid formation, and this probably relates to a propensity for scar tissue formation; if this happens in a middle ear with an Esteem, it can cause fibrotic bridges that would allow for mechanical feedback to occur. Other contraindications include hypersensitivity to silicone, rubber, polyurethane, stainless steel, titanium and/or gold. Pre-existing medical conditions or current treatment that might affect the healing process, and implantation during pregnancy are also contraindicated.
When a patient is seen for candidacy assessment for the Esteem, it is important to perform a comprehensive audiological evaluation including air and bone conduction, tympanometry, word recognition (preferably using recorded materials), and SRT. Sound field aided thresholds are nice to obtain, as well as word recognition tested at 50 dB HL and at a comfortable suprathreshold level.
Expectations Management
Guiding patients’ expectations is very important. Audiologists need to ensure that Esteem candidates do not assume that they are going to have normal hearing with the Esteem. In some cases, the Esteem may provide more benefit than the patient's current hearing aid, as we saw in the clinical trial patients. However, the Esteem cannot always match the performance or the features of a well-fit hearing aid. With any kind of implantable device, there is a risk that a patient will expect that the implant is going to cure his or her hearing loss. The fact remains that s/he has a sensorineural hearing loss, with damage within the cochlea that is likely going to result in broader auditory filters and cochlear distortion of sound that the Esteem cannot fix.
Esteem SoundFit
I want to talk now about a tool that we developed recently, called Esteem SoundFit. This is a web-based tool that is based on analysis of hundreds of clinical case histories of Esteem patients. It allows us to use a patient's baseline unaided, pure-tone thresholds to calculate recommended Esteem settings for best fit to NAL-RP (revised, profound) prescriptive targets and to estimate projected Esteem-aided thresholds. (We use the NAL-RP because the Esteem is a linear system.) This tool supports evaluation of device capabilities and limitations, relative to a given audiometric configuration. It can be useful in evaluation of patient candidacy for the Esteem and can be used to directly support patient counseling prior to referral for surgical consultation, in addition to being useful in Esteem patient activations and fittings.
Figure 15 shows a screen shot of the SoundFit page. Right now, it is a standalone web page. In the near future, it is going to be incorporated into our fitting software, which will be NOAH-based. In the current version, the app elements include an area at the top for baseline thresholds to be entered by the user. Once those pure-tone thresholds are entered, the first column will populate with recommended Esteem settings for best fit to NAL prescriptive targets. The remaining columns are for manipulating parameter settings, to see what happens (with the typical patient) if different parameter values are used.
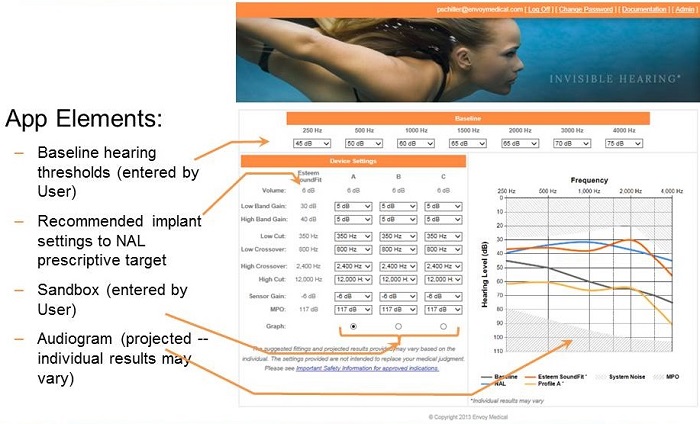
Figure 15. Screen shot of Esteem SoundFit.
The audiogram displayed on the right shows NAL target functional gain thresholds, calculated from the baseline thresholds that were entered at the top of the webpage. The orange curve displays an average Esteem patient’s functional gain thresholds for the indicated settings.
This tool can be used when you wonder if a patient is a candidate for this device. If their hearing loss is severe, you can plug their thresholds into the baseline boxes and then see how close of a match the orange curve is to the blue curve. This will tell you how close the average patient with that degree of hearing loss comes to achieving NAL target thresholds.
We have been finding that this is a very useful application. First of all, we can use it with current Esteem patients to help us determine what settings to use when programming their devices. Just as important is the value of using it preoperatively to help determine candidacy, to assess whether the device is likely to be able to provide adequate audiometric benefit to a given patient.
Reference
Hickson, L., Meyer, C., Lovelock, K., Lampert, M., & Khan, A. (2014). Factors associated with success with hearing aids in older adults. International Journal of Audiology, 53(S1), S18-27. doi: 10.3109/14992027.2013.860488.
Cite this content as:
Anderson, L. (2014, July). The Esteem® Hearing Implant. AudiologyOnline, Article 12780. Retrieved from: https://www.audiologyonline.com


