Editor’s Note: This text course is an edited transcript of a live webinar. Download supplemental course materials.
Dr. Westbrook: I will start by giving a brief overview of Oticon Medical as a company. Following that, I will include a short review of boneanchored principles for those of you who do not yet have a working knowledge about the treatment. Throughout the presentation today, you will see the abbreviation BAHS, which officially stands for bone anchored hearing system.
We will continue on to some general history and evolution of BAHS treatment methods, and then will take a look at the research that is relevant to our percutaneous BAHS treatment. Next, we will discuss other important aspects that you want to consider talking to your patients about when they are considering a BAHS treatment; we will talk about percutaneous systems specifically. Finally, I have added a brief surgery section at the end so that we can see all of the parts of the surgical system in greater detail.
Learning Objectives
At the end of this course, you will be able to identify the different types of bone conduction hearing solutions. You will be able to describe the importance of high-frequency amplification on language development, and you will be able to describe the detrimental impact of skin-drive systems on hearing performance.
History of Oticon Medical
In 2004, Patrik Westerkull began a project in bone anchored hearing systems through Otorix. This project was acquired by William Demant Holding in 2006 and subsequently, Oticon Medical was formed. In 2009, the first iteration of Ponto was launched, and that brought digitally programmable devices and the sound quality that we would come to expect from Oticon products to our bone anchored users. Within that same year, the devices received approval from the Food and Drug Administration (FDA) and also became CE marked in Europe.
Two years later, Ponto Pro Power, the first digitally programmable power sound processor, became available. Following that, the Ponto surgical system retained its universal interface, which provides freedom of choice for bone anchored patients.
In 2012, of the wide Ponto implant was introduced, which is now our standard implant, as well as the first implant and abutment system designed for tissue preservation surgery. In 2013, the Ponto Plus family of products with wireless capabilities was launched, and in the same year, we acquired the French cochlear implant manufacturer, Neurelec. We have since brought them in under the Oticon Medical brand name.
Looking into the future, our discussions continue about the path of bone anchored devices. Specifically, our BAHS business division is located in Gothenburg, Sweden. I sit in Copenhagen along with many of my colleagues within the bone anchored division and central business, while our cochlear implant facilities remain in the south of France.
The bone anchored field has progressed rapidly since Oticon Medical entered. I believe that competition is what ultimately drives innovation in our field, and over 25% of new bone anchored patients choose Ponto. Research results support the fact that two out of every three patients select Ponto if they are given a choice of their device.
BAHS Principles
Ponto is a BAHS consisting of three main parts. First, we have an external sound processor, which takes the acoustic sound signal from the environment and transforms it into vibrations. That sound processor is attached to an abutment, which is a small post in varying length dependent upon the skin thickness, and that abutment connects to small titanium implant, which is secured in the bone of the skull. The implant itself is only 3 mm or 4 mm long, dependent on the patient's bone thickness.
The Ponto system and other bone anchored devices take advantage of the body's natural ability to transmit sound through bone, and therefore they send auditory input directly into the cochlea. This occurs in a few different ways for different types of hearing losses.
Another important aspect of bone anchored technology is a natural process called osseointegration. It was first well studied and proven in the field of implant dentistry. We saw that when a titanium implant is put into bone, the bone cells begin to grow and integrate themselves onto the implant surface. Applying this to audiology, we can take greater advantage of osseointegration, because the process is making the connection between the implant and the skull bone even stronger, and in the end, it is better for transmitting sound.
How does the Ponto System Work?
The way the Ponto system works for different indications depends on the type of hearing loss. There are two main candidacy groups: conductive or mixed hearing loss, and single-sided deafness (SSD). For a conductive hearing loss, the sound processor acts as a bridge. Through bone conduction, the sound signal bypasses any dysfunction in the outer or middle ear and is delivered directly to the cochlea. For mixed hearing loss, we also must contend with some sensorineural hearing loss once we have managed to deliver the sound to the cochlea. Therefore, the fitting range for these mixed-hearing-loss candidates is that the patients need to have an average bone conduction threshold of 55 dB HL or better.
SSD patients have one working cochlea and one dead cochlea. This is a unilateral, profound sensorineural hearing loss. In this indication, Ponto is fit on the side of the dead ear and sends a sound signal to the good cochlea through bone conduction, lifting the head shadow. These patients must have normal or near-normal hearing in their good ear in order to be sure that the device will provide enough amplification to the good cochlea in the face of transcranial attenuation.
Bone Anchored Solutions
Figure 1 compares the bone conduction devices and also shows how they are related in terms of sound transmission. On the right side, we have skin drive systems, which was the industry's first idea, which we now know to be fairly simple.
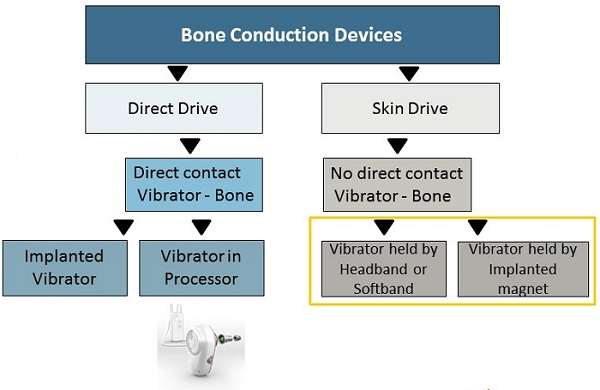
Figure 1. Direct Drive compared to Skin Drive systems.
Skin drive systems offer no direct contact between the vibrating source and the bone. Some people call skin drive indirect drive; it is initially how technology worked with conventional bone conduction hearing aids and bone conduction eyeglasses years ago. It uses the same force or method as you use to test bone conduction in a booth. It is also what a patient would experience using a Ponto on a headband or soft band.
As treatment has evolved over time, we have moved to the left branch of the tree, or direct drive. In these solutions, there is direct contact between the vibrating source and the bone, for example, the percutaneous skin penetrating Ponto system. Direct drive is well proven as the best treatment for patients who require amplification via bone conduction.
As we then moved on in time, other manufacturers have look to create transcutaneous or non-skin penetrating systems where an external vibrator is held in place by both an external magnet and an implanted magnet. The results of such devices are quite similar to what we had already accomplished in the past with headband and softband solutions.
When aided thresholds of patients measured with a bone anchored device attached to a headband are compared to bone anchored device directly on an abutment, there are statistically significant differences in these levels in the mid to high-frequency region (Verstraeten, Zarowski, Somers, Riff, & Offeciers 2008). Conclusions from this study indicate 10 to 20 dB attenuation of sound in this high-frequency region for skin drive solutions.
In Figure 2 we have a comparison of the output of a Ponto Plus device on an abutment to the output of a magnetic skin drive device. The curve has been corrected because of attenuation that is happening because of the skin drive system. The exact values of the correction are taken from the Verstraeten et al. (2008) study, and the magnetic device has lower output in the mid to high-frequency region. That is further compounded by the attenuation through the skin.
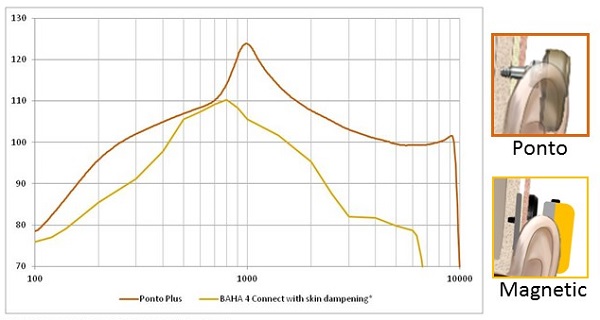
Figure 2. Sounds are attenuated in skin drive solutions, as indicated on this comparison graph (red = percutaneous; yellow = skin drive by magnet).
Output curves makes sense to use, but we have developed a schematic more relatable for patients (Figure 3). When wearing Ponto on an abutment, what patients can hear is shown in the red shaded area. Ponto can offer amplification in a wide range, which is necessary for patients to hear the sounds in their environment. To clarify, however, it does not mean that the patient cannot hear sounds above 70 dB. It means that, to the patient, the sounds will not be perceived as louder than 70 or 80 dB. A solution that attenuates due to the lack of direct vibrator to bone contact will greatly reduce the patient's perception of important speech input.
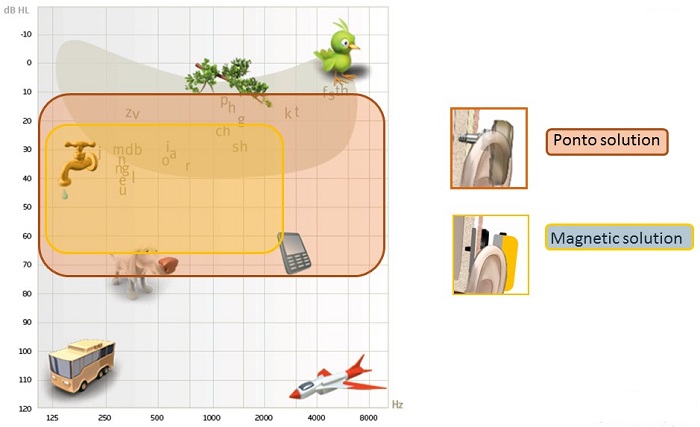
Figure 3. Patient schematic showing the range of audibility for the Ponto solution (red) and a skin drive magnetic solution (yellow).
Percutaneous BAHS Research
Having seen that the percutaneous solutions offer the best high-frequency amplification, we want to provide access to these frequencies as early as possible for children who are developing language. In one study, Andrea Pittman (2008) used a set of five nonsense words and five novel pictures which correspond to the words. This method was used to determine the learning rate under different conditions of the test subjects.
The words presented were filtered in two different ways. One group was presented with words under a typical 9 kHz bandwidth. and the other group was presented with a limited 4 kHz bandwidth. The test subjects for this study included both children with hearing loss and children with normal hearing. Learning the words in this study was defined as a 50% correct rate, and regardless of their hearing status, we see that children who completed the testing under the limited bandwidth condition took, on average, 75 trials to reach that 50% correct level, as compared to 25 trials for children in the extended bandwidth group.
They needed three times as many trials in the limited bandwidth group to learn those new words. This study underscores the importance of providing as much access to high-frequency sounds as possible for our language learning patients.
Another pediatric case study (Verhagen, Hol, Coppens-Schellekens, Snik, & Cremers, 2008) takes this concept a bit further. One child was fit with a device on a soft band at age 4 months. Following initial fitting, data was collected at age 2 years. At that time, the child was developing language at an above average rate. We would expect this with some degree of amplification.
At a follow-up visit a year later, language development scores had dropped to below average. A decision was made for implantation, which then occurred at three years and eight months. A one-month follow-up on this fitting showed a continued below-average language development score, but then the appointment following that about a month later showed an accelerated improvement in speech development after implantation. For this patient, and this is likely the case for others, the transition to a direct drive solution was what was necessary for them to maintain normal language development.
The age of fitting of amplification tells us a lot about future skills for children, from speech perception, speech production, and general language skills. The same goes for the development of the auditory system in general. The better the access a child has to relevant information earlier on in life, the better the child will do in the future. If a child is going to undergo surgery in order to improve their hearing outcomes, we need to be sure that we do document an improvement in their language development.
Emphasis of High-Frequency Amplification for Patients with SSD
The importance of amplification via percutaneous systems is definitely not limited to pediatric applications. For both adults and children with SSD, the goal that we have through providing bone anchored treatment in the first place is to lift the head-shadow effect. As this effect exists above 1500 Hz, it is only the high frequencies with which we are concerned. We do not want to amplify the low frequencies for these patients, because that might disturb the natural hearing occurring in their good, contralateral ear. In the end, that would lead to poorer performance when listening in the presence of background noise. As you have seen, percutaneous solutions are really the ones that can offer this high-frequency amplification.
Additionally, when we are sending a sound signal from the deaf side across the skull, we know that it will lose some strength because of transcranial attenuation, mainly from 2000 Hz and up. Because of this, even more high-frequency amplification is needed, and the best way to provide that is by using a percutaneous device.
Since only direct drive solutions can provide the necessary amplification, fitting a skin drive solution or indirect drive solution for an SSD patient will most likely lead to poor results. Another consideration for fitting a device with more high-frequency amplification is the increased likelihood of feedback. Within Inium feedback shield, which is a technology that we share with our sister company Oticon, Ponto devices can provide this needed amplification without worrying about bothersome feedback.
Patients with mixed hearing loss should also be considered here. Since we have to compensate for hearing loss that in their good cochlea, we must be sure that the device that we use is going to be powerful enough for them. The skin attenuation plus the additional cochlear amplification is going to leave very little headroom for this patient. Only a power bone anchored sound processor, like a Ponto Plus Power (Figure 4), can provide the necessary amplification for these kinds of patients.

Figure 4. Ponto Plus Power.
As we saw with SSD patients earlier, mixed hearing loss patients require additional amplification that might cause feedback. Ponto has an effective feedback management system, and it ensures that the prescribed gain is not cut down due to a feedback limit, that patients are not disturbed by feedback, and also importantly that they can turn up the volume on their device, if they wish.
What Patients Should Know about Skin Drive versus Direct Drive BAHS
How do you convey to your patients the importance of using a percutaneous system when they are sitting in your office? There are benefits and limitations to each of the different solutions from all manufacturers, and we have to be prepared to discuss them all with our patients. That is why we encourage audiologists to support informed decisions.
When you start down this path with your patients, you should consider at least discussing opinions and options regarding the following three popular questions. First of all, does the selected solution provide enough amplification or output? We can call this category hearing. Secondly, is the solution comfortable enough so that the patient can wear it for a full day as we recommend? This category is called comfort. Third, and probably most popular, how does this solution compare cosmetically to other solutions? We call this category cosmetic.
Hearing
The schematic in Figure 3 is used to illustrate the facts that audiologists know so well, but when you are sitting in front of a patient, it can more helpful to explain it in this way. Using a direct drive percutaneous solution like Ponto is what provides access to important speech phonemes in a wide frequency range. Switching to a skin drive solution is what greatly limits access to important sounds in your patients’ lives. In addition to sitting with an audiogram and explaining it in this way, you are now also armed with evidence about power and high-frequency gain and feedback. You should be touching upon that with your patients as well. This schematic is a great place to start the conversation.
Comfort
Patients who wear Ponto report minimal physical sensation of the device. They report that it stays securely in place once they have attached it to the abutment, and they have not reported other factors that prevent them from wearing it as recommended, which is during all waking hours. We want bone anchored patients to have the same kind of daylong benefits as conventional hearing aid patients. Figure 5 shows a comparison of wearing time between a magnetic skin drive solution (Ray, 2014) and a BAHS (Dutt, McDermott, Jelbert, Reid, & Proops 2002). These reports illustrate the difficulty with using a skin drive solution in the same way as a percutaneous solution.
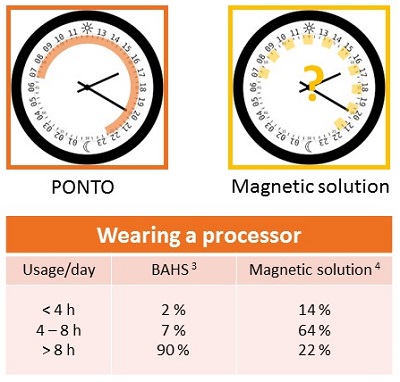
Figure 5. Daily usage and comfort comparison of the Ponto (red) and magnetic skin drive solution (yellow).
First, you can see that a third of patients reported experiencing pain when they were wearing the magnetic device. There is a high risk of retention issues whereby patients are instructed to always wear their safety line with these devices, lest they fall off and get lost. Finally, when we look at the magnetic solution, we see that only 22% of users of the system were able to wear the device for an entire day, which was defined as over eight hours. That means that 78% of patients fit with this solution are using their skin drive solution for less than eight hours a day.
When you fit a patient with a device, you would expect that they are able to wear it as you recommend. Remind them of the importance of consistent use, just as you do with conventional hearing aids, and discuss the high comfort of daily wear for a percutaneous device, like Ponto.
Cosmetic
Cosmetic aspects are likely the most talked about among bone anchored patients. We do believe that any surgery for bone anchored treatment should be the most minimally invasive as possible. That is to cause the least degree of numbness and the least amount of pain for the patient. Tissue preservation surgery for Ponto, which is what I will discuss in the next section, leaves only the small abutment post as evidence of the procedure. It is also a reversible procedure.
There is no evidence of an implanted magnet when you take the device off. You will sleep like royalty when you have removed a magnetic device. Patients should know to do an apple-to-apple comparison. Compare the cosmetic outcome when the sound processor is in use. In this case, you can see in Figure 6 that the Ponto device has the lowest physical profile.
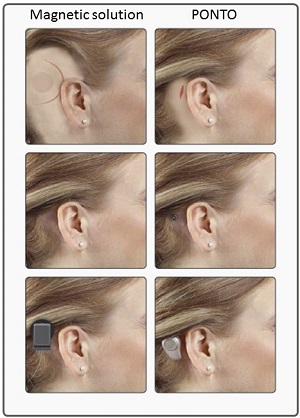
Figure 6. Cosmetic profile of a magnetic device and the Ponto.
Pediatric Patients
When it comes to pediatric counseling, these same topics that I introduced above ring true. It is important to remind parents of pediatric patients that a soft band fitting should not be delayed. Some parents wonder if the abutment solution is really the top solution, then why do we have to be bothered with fitting on soft band first? The fact is that it is the best and only choice for pediatric patients prior to the recommended age of surgery. The age of implantation in the U.S. is five years as determined by the FDA.
When the child is old enough for an implant, we know the importance of high-frequency input and output. We know the importance of wearing the device all day. We know the importance of device comfort. Development does not stop for kids once they reach that age of implantation. There is no reason to offer a surgical solution with the same audiological advantages of not even having the surgery at all.
Figure 7 provides a summary to which you can refer back. You should feel confident that fitting a direct drive percutaneous system, such as Ponto, is appropriate for both pediatric and adult populations, whether they have a conductive hearing loss, a mixed hearing loss, or if they have SSD. You have seen the advantages of fitting Ponto over a skin drive system for these groups. A skin drive system that you are fitting in the form of the headband or soft band remains the most appropriate in two cases: for pediatric patients before they reach the age of surgery and for pre-surgical demonstration purposes with adults and older children.
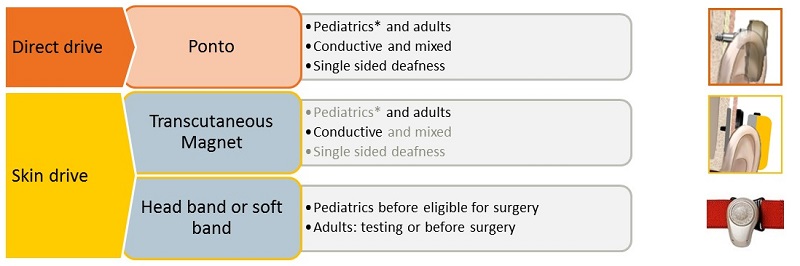
Figure 7. Summary of candidates for BAHS.
Surgical Overview
I promised you a short surgical overview for the percutaneous Ponto treatment. I am not a surgical expert, but I am very happy to answer questions that are within my scope of knowledge. In Figure 8, you can see the complete Ponto Ponto system overall. It is composed of three parts.
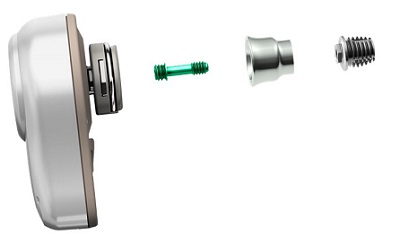
Figure 8. Ponto system.
First, we see an enlarged view of the sound processor on the far left. It is followed by a green connection screw, which is what holds the abutment and the implant together. If you look straight at a Ponto abutment, you see the top of the green screw, but it does not come out when the implant is placed. That is an easy way to identify that it is from a Ponto system. After the green screw is the abutment. In this picture (Figure 8), is a shorter length abutment, and then the abutment is attached to the implant fixture, which is shown on the far right. These parts shown are not actual size.
The standard fixture itself is called the wide Ponto implant (Figure 9). The original Ponto implant is shown in Figure 9 on the bottom right, and was was 3.75 mm in diameter; the wide Ponto implant is 4.5 mm in diameter. The wide implant is now the standard worldwide.
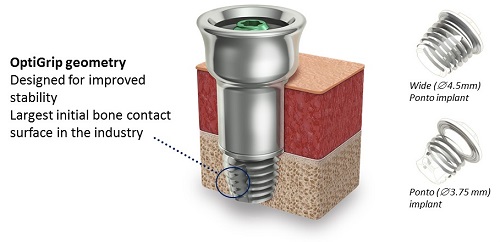
Figure 9. Ponto implant (wide and standard) with OptiGrip Geometry.
The implant is designed with OptiGrip geometry. Optigrip is used because it has the largest initial bone contact surface in the bone anchored industry. Because of the stability of Optigrip, we know that it will osseointegrate quickly, and we know it is sturdy in the bone, which is what enables longer abutments to be used.
Tissue Preservation
The Ponto system abutments are designed to support tissue preservation surgery. The OptiFit abutments have a titanium surface; that surface is the best documented solution for this type of minimally invasive surgery (Johansson, Holmberg, & Hultcrantz, 2014). OptiFit has a smooth machined surface, so the interface with the tissue during the surgery is also smooth. It is done that way to ensure that there are no pockets or pathways for bacteria to get in around the abutment. A rougher surface would provide an opportunity for bacteria to travel. Ponto abutments have been cleared by the FDA and other regulatory boards for tissue preservation surgery.
The abutment family has grown over time (Figure 10). It includes a 6 mm implant, 9 mm, 12 mm, and most recently a 14 mm length implant. On the left-hand side of Figure 10, there is a small ruler, and that is what is used by the surgeon to measure skin thickness. They typically use a needle and a small forceps, pierce the skin with the needle, hold it at the skin level with the forceps, and then pull the needle to measure the thickness of the skin against the ruler. That is used to determine which abutment size should then be selected for the best patient outcome. For example, say a surgeon measured 5 mm of skin thickness. That would mean that the patient is likely most suitable for a 9 mm abutment.
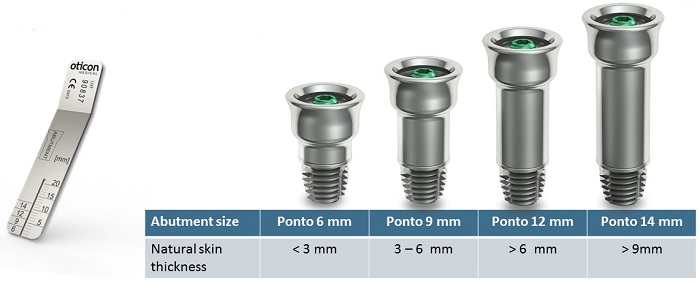
Figure 10. Ponto abutment family.
Tissue preservation surgery is a simple procedure, though many aspects of it will vary from surgeon to surgeon. It can typically be completed within 10 to 20 minutes, and most commonly it is done under local anesthesia. The wound healing time in general is usually complete within 10 days. Some surgeons will clear the patient for loading the sound processor as soon as three weeks following the surgery. Please note, however, that this timeframe is typically longer for pediatric patients.
Figure 11 shows some of the outcomes. The top left photo is during the surgery. The top right shows a sutured incision. The bottom shows post-surgical after some of the hair has grown back in around the abutment site.
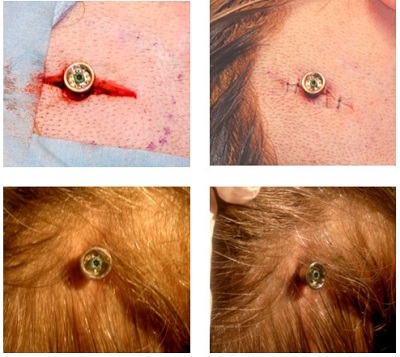
Figure 11. Tissue preservation surgery pictures during and after surgery.
The tissue preservation surgery has more than one physical method. Malou Hultcrantz published her way of doing this surgery, and that is by placing the abutment beside the incision line. An incision is made, the wound site is opened, the abutment is placed, and then skin is placed over the abutment. Then a punch is made to allow the abutment to come through. That way the sutures are alongside of the abutment. However, you can also do the procedure with the abutment placed directly in the incision line.
Ultimately, tissue preservation surgery makes a big difference for patients. Patients and studies report better cosmetic outcomes, as you can clearly see in the pictures in Figure 12. There are also fewer reports of numbness and pain. Patients report faster healing time and minimal scar tissue when compared with older methods.
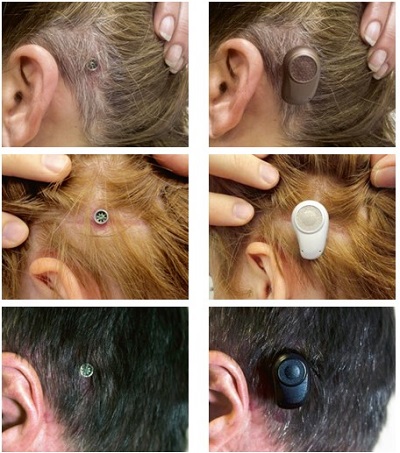
Figure 12. Tissue preservation surgery outcome pictures.
The surgeons report a faster procedure time. It is also important to note that the surgery is fully reversible without necessitating any tissue reduction to remove the implant if that became the case. That is not the same for some of the transcutaneous methods. When you do the surgery and you needed to change from a transcutaneous skin drive solution implant to a percutaneous solution, there would be some visible skin reduction on certain patients that would make the cosmetic outcome a little less attractive. The outcomes for patients that are pictured here are what tell the best story. It is not a surprise that tissue preservation surgery is the preferred method.
Questions and Answers
What is the maximum sensorineural hearing loss on a good ear that can still work with Ponto?
This goes back to SSD. The true candidacy criterion for SSD is that the good ear has normal or near-normal hearing. Technically, if we take a threshold on the good ear, and the patient has an average threshold of 20 dB or better but does have a little bit of high-frequency hearing loss, they are still a candidate. If you are going to fit this patient with a percutaneous system for SSD and they are showing a little bit of high-frequency hearing loss, I would definitely use a power device. We also have to deal with our transcranial attenuation to make sure the sound gets there in the first place. That high-frequency hearing loss cannot be so severe that it pulls down the average hearing threshold in the good ear below 20 dB.
How far can that hearing loss be pushed out? Does borderline moderate sensorineural hearing loss qualify?
The answer to that is no. Patients with more severe losses will be better candidates for BI-CROS systems, because we cannot guarantee that the amplification with BAHS. We need to make sure that we are sending a strong enough signal to the good cochlea where it will be heard. If they have too much hearing loss in the good cochlea, it will not be heard.
Can you speak to bilateral implantation for a child with bilateral atresia? Do you need two processors implanted if one device will stimulate both ears?
That is a great question. A device on one side will, to some degree, stimulate the opposite cochlea. Bilateral implantation and a promise of bilateral hearing is reserved for patients who have relatively symmetrical bone conduction thresholds between the two ears. The problem with implanting just on one side is that the sound is going to favor the ear that it is closer to. We do get attenuation in this case as we would with SSD, because the sound signal has to travel from the side of the implanted ear to the opposite cochlea. It is not going to reach the opposite cochlea to the same degree. We would definitely suggest using two devices, obviously on a soft band before implantation if possible, and then implant two devices.
Do others manufacturers use tissue preservation surgical procedure?
Yes, other manufacturers support tissue preservation surgery, but also the Ponto system was specifically designed to support this tissue preservation surgery. That is why the implants were made wider, in order to support a longer abutment. The longer abutments make it possible to do tissue preservation, because you do not have to reduce any of the skin thickness. Ponto is definitely made and cleared for tissue preservation.
References
Dutt, S. N., McDermott, A. L., Jelbert, A., Reid, A. P., & Proops, D. W. (2002). Day to day use and service related issues with bone anchored hearing adis: The Entific medical systems questionnaire. Journal of Layngology and Otology, 116(28), 20-28.
Johansson, M., Holmberg, M., & Hultcrantz, M. (2014). Bone anchored hearing implant surgery with tissue preservation – A systematic literature review. Oticon Medical white paper, M5210.
Pittman, A. L. (2008). Short-term word-learning rate in children with normal hearing and children with hearing loss in limited and extended high-frequency bandwidths. Journal of Speech, Language and Hearing Research, 51(3), 785-797. doi: 10.1044/1092-4388(2008/056)
Ray, J. (2014). Outcomes and audiological perspective of transcutanous (Attract magnetic) implants. Presentation at BAHA Professionals Meeting, Norwich, England.
Verhagen, C. V., Hol, M. K., Coppens-Schellekens, W. Snik, A. F., & Cremers, C. W. (2008). The Baha Softband a new treatment for young children with bilateral congenital aural atresia. International Journal of Pediatric Otorhinolaryngology, 72(10), 1455-1459. doi: 10.1016/j.ijporl.2008.06.009
Verstraeten, N., Zarowski, A J., Somers, T., Riff, D., & Offeciers, E. F. (2008) Comparison of the audiologic results obtained with the bone-anchored hearing aid attached to the headband, the testband and to the ‘snap’ abutment. Otology & Neurotology, 30(1), 70-75. doi: 10.1097/MAO.0b013e31818be97a
Cite this Content as:
Westbrook, J.L. (2015, September). The Ponto System and benefits of percutaneous BAHS. AudiologyOnline, Article 15188. Retrieved from https://www.audiologyonline.com.


