Otosclerosis is a common condition affecting the bone of the otic capsule in 7.3% of Caucasian males and 10.3% of Caucasian females. The condition is rare in non-Caucasians, except East Indians, in whom the incidence is approximately the same as persons of northern or central European origin.
The stapes appears fixed in only 12.3% of temporal bones with histopathologic evidence of otosclerosis. Otosclerosis causes hearing loss in 0.3% of the general population. Two-thirds of patients treated for otosclerosis are women. About 75 percent of all cases are bilateral.
Otosclerosis often starts in an area anterior to the stapes footplate called the fistula ante fenestrum but can occur in any part of the otic capsule. The second most common site of origin is the round window.
Histologically, otosclerosis is characterized by 2 phases: 1) otospongiosis; and, 2) otosclerosis. In otospongiosis, affected bone becomes hypervascular and osteoclasts and osteolytic osteocytes cause enlargement of the vascular spaces of the bone. After otospongiosis, bone deposition characterizes the otosclerosis phase.
The term cochlear otosclerosis is used to describe the sensorineural hearing loss seen in some patients with otosclerosis. This process can result in profound sensorineural hearing loss.
Photo One shows the CT of a normal cochlea. Photo Two shows the temporal bone CT of a woman with bilateral profound sensorineural hearing loss due to otosclerosis. The patient eventually underwent cochlear implantation with satisfactory results. During surgery it was noted that otosclerosis had filled the basal turn of the cochlea and obliterated the round window.
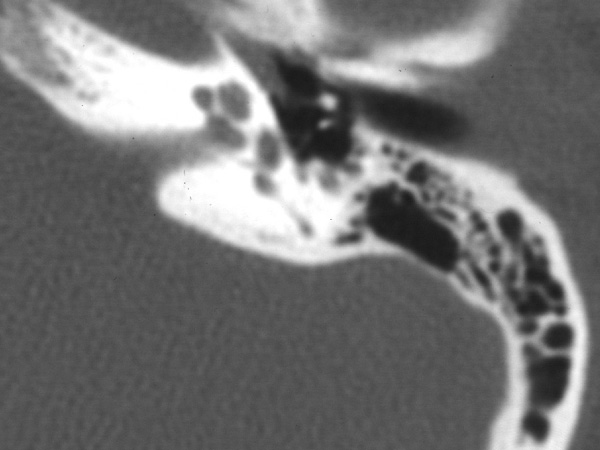
Photo One: normal cochlea
The progression of otosclerosis can be retarded, but not reversed. Calcium Fluoride (Florical) has been used to mature the foci of otospongiosis. Florical can cause fetal problems and therefore should be used with caution in women of childbearing age.
Etiology:
While the etiology of otosclerosis remains unknown, there are two main theories regarding its origin; genetic and viral.
- Genetic. Otosclerosis seems inherited in approximately 50% of cases. It may be inherited as an autosomal dominant trait with 25-40 percent penetrance and is more common in females1. Hearing loss associated with otosclerosis has long been noted to accelerate during pregnancy in some women.
- Viral. Electron microscopy has demonstrated structures similar to viral nucleocapsids in cells of otospongiotic lesions2. Immunohistochemical studies revealed measles virus nucleocapsid protein in cells associated with otospongiosis.(McKenna 1986)3 Polymerase chain reaction (PCR) studies have supported these observations and also found anti-measles IgG in the perilymph of patients with otosclerosis4.
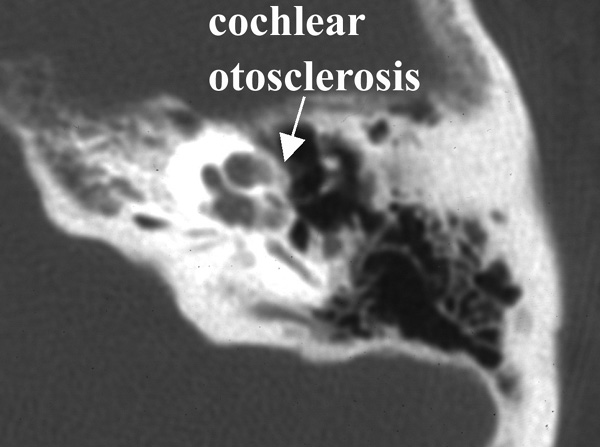
Photo Two: cochlear otosclerosis
History:
The history of the treatment of otosclerosis is rich in stories of serendipity and of treatments forgotten and rediscovered. In 1704, Valsalva first described stapes fixation as a cause of hearing loss5. In the mid-1800s, a young man with otosclerosis was noted to have hearing improvement following a skull fracture6. After he died of complications from the head injury, post-mortem examination of his temporal bones revealed a fracture through the horizontal semicircular canal. Kessel, who described the case, attempted to create a similar fistula in patients with otosclerosis using a hammer and gouge. In 1876, Kessel removed the stapes and covered the oval window with scar tissue. These early attempts at treatment failed.
Ménière first reported mobilization of the stapes in 18427, but it was not until the late 19th century that stapes surgery became more common in Europe with advances in asepsis, hearing testing (tuning forks) and discovery of the X-ray. However, frequent complications, including meningitis, and transitory hearing improvement due to refixation of the stapes and closure of the fenestration led stapes mobilization and stapedectomy to be condemned as dangerous and useless by the end of the 19th century. Throughout the late 19th and early 20th centuries, dry catarrh was the term used to describe otosclerosis8.
In 1923, Holmgren first performed horizontal canal fenestration in the modern era, covering the fistula with mucoperiosteum910. His associate, Nylen, developed a surgical microscope for the operation.
Throughout the ensuing 25 years, variations of the horizontal canal fenestration were performed in Europe and the United States. From its outset, complete closure of the air-bone gap never occurred with fenestration and results frequently deteriorated over time due to closure of the fenestrum. Even though the last fenestrations were done in the late 1950s, audiologists and otolaryngologists still encounter patients who had fenestration operations. Only an otolaryngologist or otologist should perform cerumen removal and maintenance of the post-operative mastoid cavity of these patients, due to the risk to the exposed labyrinth.
A century after it was first performed, stapes mobilization again came into vogue in the early 1950s after Rosen reported his results11. Air-bone gap closure was superior to the results achieved by fenestration, but the limitations of mobilization were soon recognized as refixation and recurrence of conductive hearing loss in a substantial number of patients. While mobilization is still occasionally performed, it has now been replaced by stapedectomy and stapedotomy.
In 1956, John Shea reported his early experience with stapedectomy. During his operations, Shea removed the entire stapes and covered the oval window with a vein graft and used a nylon prosthesis from the incus to the graft12. Subsequent refinements by Shea and others lead to the use of tragal perichondrium, fascia, and gelfoam to seal the oval window and various alloplastic materials such as wires and bucket-handle prostheses (that have a wire bale that loops over the incus) to reconstruct the ossicular chain.
In the early 1970s, surgeons began creating small holes (fenestrum) in the oval window (referred to as a 'stapedotomy') and placing a piston within the hole to reconstruct the hearing mechanism. Since then, stapedotomy has become common although many respected and competent surgeons continue to perform variations of total or partial footplate removal.
A variety of tools, including a diamond microdrill or a laser, are now used to create the fenestrum. The advantages of the microdrill include the speedy creation of a perfectly round fenestrum created to the size of the prosthesis, and the ability to work rapidly through thick footplates. The advantages of the laser include the potential for reduced trauma to the vestibule and the ability to create a fenestrum in the face of inadvertent mobilization of the footplate. In reality, both tools are well suited to the task and equally able to achieve excellent results in qualified hands.
Patient Selection:
Although there have been published series reporting hearing results in patients with air-bone gaps less than 15 - 30dB, most surgeons, including the author, recommend non-operative treatment in patients with 'small' air-bone gaps. The primary reason for this is that unless complete closure of the air-bone gap is achieved many patients with small air-bone gaps who have surgery will simply not obtain enough subjective benefit to feel the surgery was worthwhile. Nonetheless, patient selection varies from surgeon to surgeon and remains part of the doctor-patient relationship. Carefully informing patients of potential risks, benefits, and alternatives of treatment is critical in ensuring an outcome satisfactory to all concerned.
Surgical Technique:
A variety of methods have been used to perform stapedotomy and stapedectomy. It is the preference of the author to use a laser beam delivered through a micromanipulator. Other surgeons use a microfiber to deliver the laser beam to the ear. Slightly more than 50% of cases are performed under local anesthesia. A tympanomeatal flap (Photo Three) is elevated in standard fashion (Photo Four). With a bone cucurette or a microdrill, the scutum is excavated to expose the oval window, The ossicular chain is palpated to conform stapes fixation before dividing the incus-stapes joint and sectioning the stapedius tendon (Photo Five). The posterior crus of the stapes is then partially vaporized with the KTP laser. The superstructure of the stapes is then fractured toward the promontory and removed. Using bursts of the laser a 0.7 - 1.0mm fenestrum is made in the stapes footplate. (Photo Six) No irrigation is used; however, time is allowed between bursts for tissue cooling. Bone fragments are removed with a 0.3mm McGee oval window rasp. The fenestrum is measured with a 0.6mm rasp. For obliterative cases, the microdrill is used with a 0.7mm diamond burr to thin or fenestrate the footplate. A 0.5 or 0.6mm diameter McGee piston (platinum wire and stainless steel barrel - Photo Five) is placed in the fenestrum and the crook crimped over the incus. (Photo Seven) If the fenestrum exceeds 0.7mm in diameter, leaving a gap between the margin of the fenestrum and the barrel of the prosthesis, the area is loosely packed with subcutaneous tissue harvested through a small incision made on the posterior concha. If there is only a tiny gap between the barrel and the margin of the fenestrum, a blood seal is used. Patients are discharged after a brief observation period in the outpatient surgery suite.

Photo Three: tympanomeatal flap incision location

Photo Four: tympanotomy

Photo Five: cut stapedius tendon

Photo Six: fenestrum

Photo Seven: prosthesis in fenestrum
The use of the laser is not essential to achieving good results. Sedwick, et al., found in reviewing 550 patients operated on by different surgeons at the House Ear Clinic that microdrill and laser techniques were equally safe and effective in the creation of the fenestra13. They also found that post-operative high frequency hearing loss for the large fenestra techique was significantly greater than with the small fenestra but concluded that this was probably not clinically significant.
In reviewing his personal surgical experience, the author found there was no significant difference in the hearing results obtained with 0.5 versus 0.6mm pistons.
Anatomical Variants:
A variety of anatomical issues may challenge the stapes surgeon.
A. Obliterative otosclerosis:
In the author's experience, a markedly thickened or obliterated stapes footplate is encountered in 8% of cases. Other series have reported an incidence from 7 - 11%. While the first case the author encountered was managed solely with the laser, experience demonstrated this technique to be tedious. The potential for heating the tissues with the laser when dealing with a thick footplate seemed likely to outweigh the benefit of the otherwise relatively atraumatic technique. For this reason, the author now uses the microdrill to thin the footplate and either finishes fenestrating with the laser or perforates the footplate solely with the microdrill.
In such cases, closure of the Air-Bone gap to 10dB was achieved in all but the first obliterative case encountered.
B. Perilymph 'gusher' or ' oozer':
Perilymph 'gushers' are due to an abnormally patent cochlear aqueduct, or an incomplete partition between the subarachnoid space and the vestibule at the lateral end of the internal auditory canal14. Gushers are luckily rare and, once encountered, are not soon forgotten. In about 50% of cases, stapes gusher will result in profound sensorineural hearing loss.
First described in 197115, a rare, X-linked condition characterized by bilateral conductive hearing losses and perilymphatic gushers has now been described in several families. Patients have a distinctive enlargement of the internal auditory canal on CT scanning. Surgeons should be particularly watchful in young boys with bilateral mixed hearing losses. Female carriers of the gene may manifest milder hearing losses. In the author's experience, the one 'oozer' encountered so far was in a girl with congenital bilateral conductive losses. A CT obtained after successful stapedotomy showed no obvious abnormality.
C. Persistent stapedial artery:
A persistent stapedial artery is a congenital anomaly reported to occur in 1 of 5,000 - 10,000 ears16. In this anomaly, the remnant of the dorsal end of the second aortic arch, the stapedial artery, courses through the space between the crura of the stapes (the obdurator foramen) after arising from the carotid artery. In this position, the vessel obscures the stapes footplate.
The stapedial artery first appears in the tissue that forms the stapes at about the fifth week of fetal life and normally atrophies during the third month of development.2 The arch of the adult stapes is partly formed around the stapedial artery in a manner similar to the 'lost wax' method of jewelry casting. After ascending the promontory in a bony canal just lateral to the round window and basal turn of the cochlea and passing through the obdurator foramen, it turns anteriorly to join the facial nerve and ends by replacing the middle meningeal artery or by branching into the supra- or infraorbital or mandibular arteries17.
Photo Eight shows an intraoperative photograph taken by the author of this otologic rarity during stapedotomy. During the surgery, the artery is displaced inferiorly to expose the footplate between the facial nerve and the artery.

Photo Eight: stapedial artery
D. Narrowing of the ear canal:
This fairly common problem varies from mild narrowing of the external meatus that limits the exposure of the eardrum to frank stenosis by exostoses or large suture lines. Narrowing of the ear canal can be managed by meatoplasty. While meatoplasty can be performed at the same time stapedotomy is done, for very narrowed canals, I prefer to perform meatoplasty and then perform stapedotomy after the meatoplasty has healed.
Complications
A. Perilymphatic fistula:
By its very nature, stapedotomy or stapedectomy creates a fistula between the perilymph-containing vestibule and the middle ear. What is surprising is how rarely this surgical fistula results in symptoms associated with fistula, namely hearing loss and vertigo. A fistula is the cause of failure in 9 - 10% of cases.
The vertigo associated with a perilymphatic fistula is typically triggered or increased by pressure. If symptoms do not resolve with bed rest, surgical re-exploration is warranted.
B. Dysgeusia (altered taste sensation):
The chorda tympani courses anteriorly from the facial nerve, crossing lateral to the long process of the incus and medial to the malleus to enter the iter chorda anterior - a tiny hole anterior to the malleus. In its normal position, the chorda is at risk of injury during stapes surgery since displacement is usually required to gain the access to the oval window required to safely perform the surgery.
Injury to the nerve occurs in up to 30% of cases. Symptoms include a dry mouth, sore tongue, and a metallic taste. Symptoms usually subside in 3 - 4 months. Stretching the chorda is more likely to result in symptoms than simply dividing the nerve.
The author makes every effort to spare the nerve to avoid taste disturbance.
C. Tympanic membrane perforation:
This complication arises occasionally. It most commonly occurs when the surgeon, attempting to elevate the tympanic membrane annulus from its groove, accidentally perforates the drum. It can also occur as the result of a post-operative infection. In all but a few cases, these typically small perforations heal uneventfully. Only a few will require surgical closure.
D. Infection:
Luckily rare, a post-operative infection after stapedotomy can lead to profound hearing loss and, in rare cases, meningitis. The latter occurs when bacteria transgress the vestibule to enter the CSF-containing subarachnoid space.
E. Profound hearing loss:
The risk of profound hearing loss is 0.6 - 3% in large stapedectomy series. The risk is considerably higher in revision stapes surgery. The chief cause of post-stapedectomy hearing loss is surgical trauma, especially extensive drilling18.
F. Dislodgement of incus:
Two situations that can lead to inadvertent dislodgment of the incus are: 1) during removal of the bony rim of the annulus to expose the ossicles; and, 2) during placement of the prosthesis on the long process.
G. Erosion of incus:
While over-aggressive crimping of the crook of the prosthesis can fracture the incus, more commonly the long process of the incus erodes over time, causing the prosthesis to loosen and disarticulate, leading to conductive hearing loss. This erosion may be due to disruption of the blood supply to the distal long process of the incus . It may also be due to erosion by a bucket-style prosthesis or by over-crimping a piston with a wire-crook. Photo Nine, taken during revision stapes surgery, shows a bucket-style prosthesis that eroded the incus, causing the prosthesis to fail.

Photo Nine: displaced prosthesis
H. Floating footplate:
In manipulating the stapes, the footplate may occasionally be dislodged if it is only partially fixed. In stapedectomy, this may create a problem if the footplate becomes depressed into the vestibule and cannot be safely removed. In laser stapedotomy, the floating footplate can be fenestrated and a piston placed as usual.
I. Facial weakness:
Facial weakness is rare complication of stapes surgery. It is most often temporary and due to the effects of local anesthesia. In these cases, it improves over a few hours. In some cases, it may be delayed and may be the effect of traction on the chorda tympani, heating of the nerve by the laser, or reactivation of herpes simplex virus-1.
Results:
The author currently explains to patients considering stapes surgery that closure of the air-bone gap to within 15dB is achieved in nearly 100% of cases, within 10dB in 90% of cases within 5dB in 50% and complete air-bone gap closure in 30%. These statistics and results are based on the author's personal experience. There have been no profound hearing losses to date. These results are similar to clinical outcomes experienced by other otologists.
__________________________________________________________________________________
1 Altmann F. The inner ear in genetically determined deafness. Acta Otolaryngol (Stockh) 1963; 187:1-39.
2 McKenna MJ, Mills GB, Galey FR, Linthicum FH. Filamentous structures morphologically similar to viral nucleocapsids in otosclerotic lesions in two patients. Am J Otolaryngol 1986; 7:25-8.
3 Arnold W, Friedmann I. Presence of virus specific antigens (measles, rubella) around the active otosclerotic focus. Laryngol Otol 1987; 66:167-71.
4 Niedermeyer HP, ArnoldW. Otosclerosis: A measles virus associated inflammatory disease. Acta Otolaryngol (Stockh) 1995; 115:300-3.
5 Valsalva AM. Tractus de avre humana. Bologna, 1704 cited in: House HP. The evolution of otosclerosis surgery. Otolaryngology Clin N Am 1993; 26(3): 323-33.
6 Kessel J. Uber das mobilisieren des steigbugels durch ausschneiden des trommelfelles, hammers und amboss bei undurchganikeit der tuba. Arch Ohrenheilkd 1878; 13:69-88. Cited in: House HP. The evolution of otosclerosis surgery. Otolaryngology Clin N Am 1993; 26(3): 323-33.
7 Ménière P. De l'exploration de l'appareil auditif, ou recherches sur les moyens prospres à conduire au diagnostic des maladies de l'oreille. Gaz Med Paris 1842; 10:114-7. Cited in: House HP. The evolution of otosclerosis surgery. Otolaryngology Clin N Am 1993; 26(3): 323-33.
8 Politzer. Surgery of the Ear. Translated and edited by Ballin MJ and Heller CJ. (Philadelphia; Lea & Febiger) 1909.
9 Holmgren J. Some experiences in surgery of otosclerosis. Acta Otolaryngol 1923; 5:460-6.
10 Holmgren J. The surgery of otosclerosis. Ann Otol Rhinol Laryngol 1937; 46:3-12.
11 Rosen S. Restoration of hearing in otosclerosis by mobilization of the fixed stapedial footplate. An analysis of results. Laryngoscope 1955; 65:224-269.
12 Shea J Jr. Fenestration of the oval window. Ann Otol Rhinol Laryngol 1958; 67:932-51.
13 Sedwick JD, Louden CL, Shelton C. Stapedectomy vs stapedotomy. Do you really need a laser?.Arch Otolaryngol-Head Neck Surg 1997; 123:177-80.
14 Jackler RK.
15 Nance WE, Setleff R, McLedd A, Sweeney A, Cooper C, McDonnell F. X-linked deafness with congenital fixation of the stapedial footplate and perilymphatic gusher. Birth Defects 1971; 7:64-9.
16 Schuknecht HF: Pathology of the ear, 2nd ed. Baltimore: Lea & Febiger, 1993, p. 154.
17 Marion M, Hinojosa R, Khan AA. Persistence of the stapedial artery: a histopathologic study. Otolaryngol Head Neck Surg 1985. 93:298-312.
18 Schuknecht HF. Sensorineural hearing loss following stapedectomy. Arch Otolaryngol 1962; 54:336-347.

