This text course is an edited transcript of a Cochlear webinar on AudiologyOnline.
Learning Outcomes
After this course, participants will be able to:
- List the benefits of acoustic hearing.
- Describe the expanded indications for the Nucleus Hybrid.
- Outline the steps to programming a Nucleus Hybrid Device.
Why Hybrid?
Cochlear has led the way with the design, the development, and approval of the first Hybrid Implant System available in the U.S.
So first, why Hybrid? What are we, as audiologists, aiming for when we start a treatment plan for an individual with hearing loss? The first goal is audibility since it's a prerequisite for speech understanding. The goal of Hybrid Hearing is to provide audibility in the speech range of 125 to 8000 Hz.
The Hybrid was specifically developed for those in-between candidates who no longer benefit from hearing aids, especially in difficult listening situations such as background noise, and who do not meet the indication for a traditional cochlear implant (CI). These people have low frequency hearing with the ability to understand high-contextual sentences, especially in a quiet sound booth, and so performance is too good for traditional CI candidacy. The Hybrid Implant System provides the best of both worlds. It combines electric hearing where they need it in the high frequencies, and continued access to low frequency acoustic information in the implanted ear and via the contralateral ear. Electric stimulation, or cochlear implant hearing, is critical for improved speech understanding for people with significant high frequency sensorineural hearing loss. We know that hearing aids aren't capable of amplifying the speech range enough for severe to profound thresholds to be within the speech range. At the same time, we know that low frequency information provides benefits such as enhanced sound quality, improved understanding in background noise, fundamental frequency cues for both pitch and vowel discrimination, and interaural timing difference cues. You can achieve some of these benefits in a bimodal fitting condition as these people have a lot of low frequency hearing in both ears. Our Smart Hearing Alliance with GN ReSound was created to maximize bimodal fittings. However, why not try to optimize hearing in both ears to allow the brain to access binaural redundancy or summation?
Pivotal Clinical Trial
Before I discuss the Pivotal Clinical Trial, I would like to provide a quick summary of the different phases of Food and Drug Administration (FDA) regulated studies. New medical devices are essentially studied in phases, regulated by the Food and Drug Administration. The first phase is called a Pilot or Feasibility study. In this phase, a device is implanted in a relatively small number of people in order to study initial safety and performance information in a controlled setting. A Pivotal study is the next phase, and this involves a larger population. The goals in a Pivotal Study are to determine the safety and effectiveness of the device. Ultimately, the results from a Pivotal study provide evidence required by the FDA to consider approval of the device and bring the technology to market. This Pivotal Clinical Trial is what brought Hybrid to market. A Post-approval study is the final phase for medical devices, which gathers long-term data in a broader population. We'll get back to post-approval studies later in this talk
Results of our Pivotal Trial led to FDA approval of the Hybrid technology and expanded the indication to serve a previously underserved group of people.
Let me start with some background information. Researchers at the University of Iowa including Dr. Bruce Gantz helped to pioneer this entirely new category of cochlear implant electrode arrays. Hybrid implant studies have been going on for over 15 years, with various designs and implementations that eventually evolved into the Nucleus Hybrid L24 Implant System.
FDA Approval - March 2014
Implantations with the L24 as part of this Pivotal Trial began in 2008, and 50 adult subjects were included. After a detailed review of the study data with a primary end-point at six months post-activation, and then a panel meeting to discuss these results, the FDA approved the Hybrid L24 Implant System in March of 2014. The approval was based on the data proving that the device was safe and effective in treating hearing loss in those from whom it was intended. It's important to note that FDA approval wasn't just the implant itself. There are other key points that I want to review.
First Hybrid system implanted in the US. First cochlear implant labeled for potential preservation of residual hearing. We're now implanting a group of people with a potentially better neural substrate, or a healthier ear, if you will, and they may present with some useful low frequency acoustic thresholds. This was the first cochlear implant to have FDA approved labeling for the potential preservation of residual hearing, based on data collected in the Pivotal Study.
First indication using CNC words. Because we're implanting people who may be able to get by on the telephone, as an example, we have to challenge them in the candidacy phase. We do that using CNC words, and this is the first time an indication has been built around a word score rather than a sentence score. Sentences provide contextual clues, which may be especially useful to people with a lot of residual hearing. You may have had patients tell you that testing in the sound booth doesn't represent how they hear in the real world. While we haven’t solved this problem entirely, using single words to test candidacy brings us closer to the end goal of determining true performance in the patient's real world environments.
First integrated Electric + Acoustic Sound Processor. Finally, this approval also brought us the first completely integrated electric and acoustic sound processor, the Nucleus 6.
Pivotal Study Details
The Pivotal Study was a multicenter design with 14 surgeons across 10 implant centers. Fifty adult subjects were implanted and the data that was collected at six months post-activation provided the safety and efficacy information required by the FDA. While some sites implanted more study subjects than others, there was a nice mix with nine out of the 10 sites implanting at least three subjects and the 10th site implanting one. This is important because it helps us to know that the data collected are not due just to the skills of one particular surgeon or site, but rather, generalizable across sites.
Figure 1 shows the key demographic characteristics of our 50 subjects.
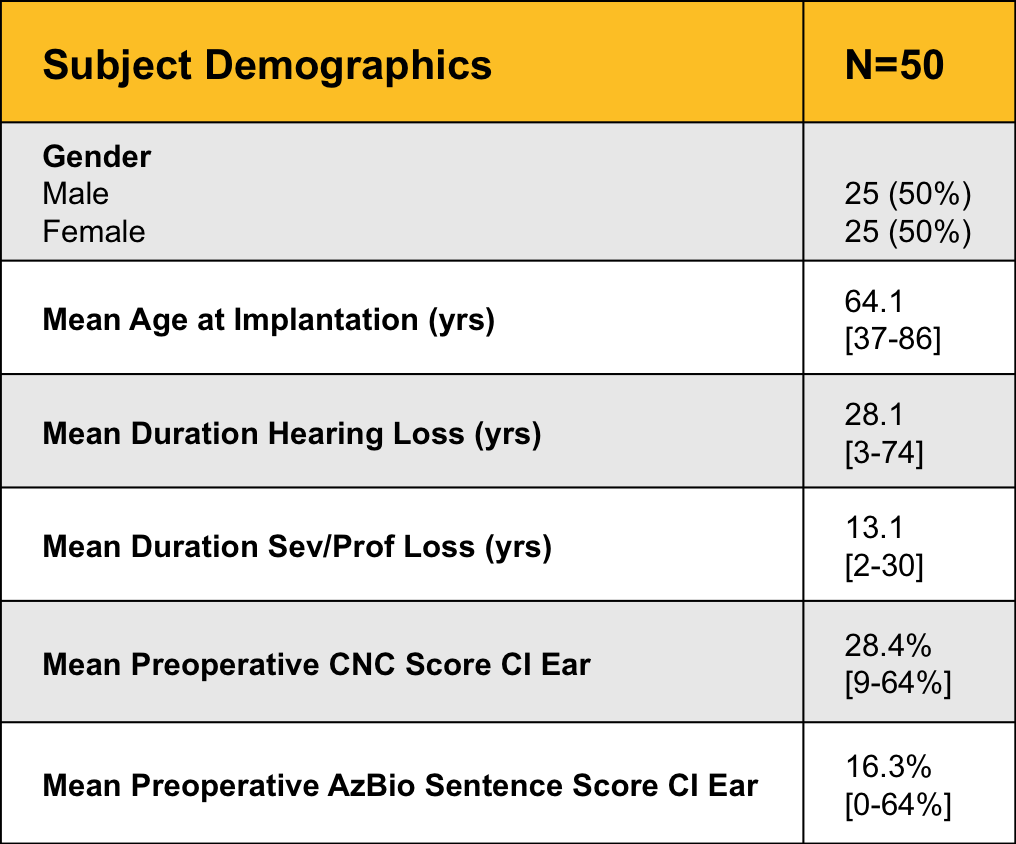
Figure 1. Subject demographics.
There was an even split of men and women, with a mean age at implantation of 64.1 years, and a mean duration of hearing loss of 28.1 years. Because this was an adult study, our lower age limit was 18 years, but we did not have an upper age limit. Note that while the mean age at implantation was about 64 years, the median age was 68 years - that means that half of the subjects were under 68 years and half were more than 68 years. It’s important to remember that age is by no means a limiting factor for selection criteria. Just as is the case in traditional cochelar implants, older people benefit from implantable technology. Just keep in mind that age can result in a higher degree of variability in study results. Other studies you may see have involved considerably younger populations, and this is just an important factor to keep in mind when you review data.
Figure 2 shows the FDA approved indication for the Hybrid L24 Implant System, resulting from the trial.
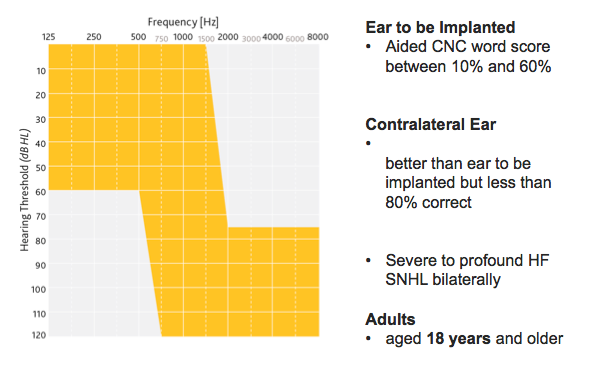
Figure 2. Hybrid L24: An expanded indication.
These are the minimum criteria the FDA considers imperative for application of the Hybrid solution to a candidate. Because we used CNC words in the clinical study, for candidacy we look at an aided CNC word score between 10% and 60% in the ear to be implanted, and up to 80% in the opposite ear. Audiometrically, candidates have a precipitously sloping high frequency sensorineural hearing loss bilaterally.
The device is indicated for adults (age 18 years and older). In the last 10 to 20 years, the indications for implantation have undoubtedly expanded. The Hybrid L24 Implant System indication opens up the discussion of treating both ears and assessing the whole patient. There are other candidacy considerations outside of these labeling indications such as duration of hearing loss, stability of loss, etiology, general health, lifestyle, communication needs, and others. Factors such as these should be considered for all potential candidates for implantable technology by the implanting team.
We are often asked about borderline candidates. What do you do with someone who meets traditional CI indications - should they get a traditional CI or a Hybrid? Well remember that theHybrid Implant is intended for candidates who don't meet the traditional CI indications, and those who truly fall in between the two technologies are pretty rare. The Hybrid L24 Implant System is serving a previously underserved population.
Key Findings from Pivotal Study
From the FDA approval in 2014, came both an established hearing preservation claim, along with documented performance outcomes, which can be found in Roland, Gantz, Waltzman and Parkinson (2016) in the journal Laryngoscope. The article is open access and available here.
These documented claims are what assist us in counseling candidates on the Hybrid indication today. Published results demonstrate:
- Significant improvement in speech understanding in quiet and noise
- Expanded indications for individuals with severe high frequency hearing loss
- Increased satisfaction, consistent with speech perception results
Recent Updates
Reimbursement
Now I will discuss some things we have been working on since FDA approval in March of 2014, beginning with reimbursement. In 2014, when we received the Food and Drug Administration approval for the first Hybrid Hearing device available on the market, there was limited access to the implant system for those with commercial insurance coverage. This is often the case with new technology. FDA approval does not equate to or guarantee insurance coverage. Once a device is available on the market, insurers gather their own data, including, of course, labeling information provided by the FDA. Insurers then decide whether or not this is a benefit they want to extend to their plan participants. It takes time to have those discussions and to demonstrate the need and the benefits.
Since August 2016, we've seen a significant shift in insurance coverage for the Nucleus Hybrid Implant System. Cochlear's been working with physicians and audiologists to advocate for insurance coverage of this hearing solution. At the time this course was presented, Anthem and 20 other independent Blue Cross-Blue Shield plans have updated their cochlear implant coverage policies to include coverage of the Cochlear Nucleus Hybrid System. Because of Cochlear's efforts along with those of the medical and audiologic community, more than 142 million people now have access to the Cochlear Nucleus Hybrid Implant System, and it is a covered treatment option for certain patients in all 50 states. Based on the current momentum, we hope to see other payers update their policies to include coverage for the Hybrid Implant system. Regarding Medicare, Hybrid is covered just like a traditional cochlear implant. CMS’s candidacy criteria are a little bit more restrictive than many commercial providers but for those who do meet the criteria, they’re covered.
We know that one important step toward that end is for payers to receive requests for pre-authorization. It helps them to understand the needs of their customers and this is where Cochlear's reimbursement team is focused.
From a clinical standpoint, we recommend that you identify and evaluate potential candidates for the technology regardless of their commercial insurance coverage. Let Cochlear's Otologic Management Services (OMS) help to request the approval. OMS is Cochlear's pre-authorization team and is part of our reimbursement department. As long as your candidate meets the on-label FDA candidacy criteria, then we can help. As with any new category of devices, insurance payers want to see a large body of evidence and high consumer demand for a product. Don't shy away from a clinical recommendation because of the potential for a denial. Our reimbursement team has a high approval rate for Hybrid pre-authorization and a good record for overturning denials. Cochlear's OMS team is available at 1-(800)-633-4667.
Keep in mind that patients you've seen in the past who were denied coverage because the Hybrid was still considered investigational, may very well have coverage now. It is worth looking into for those individuals again. If you get a denial, send it to OMS. The more information that OMS has in terms of who the candidates are and who's requesting a Hybrid, the more weight we have to try to change the insurance coverage decisions.
Acoustic Component
Another thing we've been working on since the Hybrid L24 approval is the use of the Acoustic Component with the Nucleus 6 Processor.
Let me discuss the difference between the Hybrid Implant and Hybrid Hearing. Figure 3 shows a Cochlear Nucleus 6 Sound Processor which has undergone a simple transformation to a Cochlear Hybrid Hearing sound processor by adding the Acoustic Component.
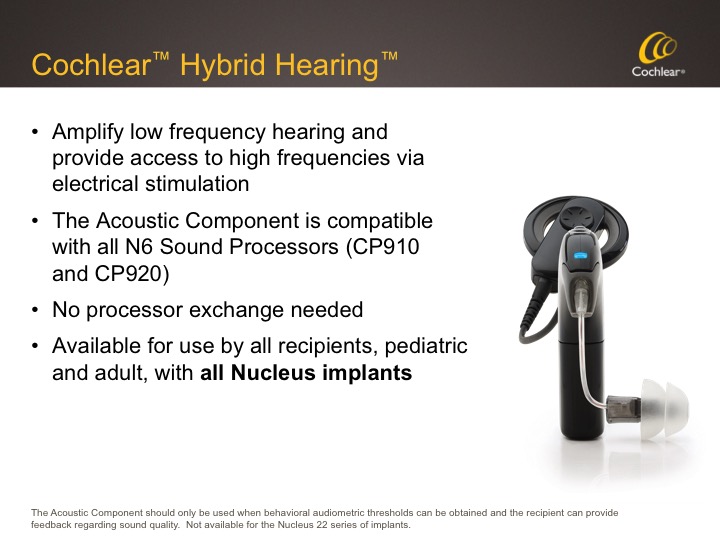
Figure 3. Cochlear Hybrid Hearing.
The goal of Hybrid Hearing Hybrid Hearing is to amplify low frequency hearing via the Acoustic Component, and provide access to high frequencies via electrical stimulation.
The Acoustic Component can be used for any Nucleus 6 user whose behavioral audiometric thresholds are within the fitting range, and who can provide feedback regarding sound quality. This is a relatively new approval for Cochlear. Previously, we were restricted to the use of the Acoustic Component on Hybrid L24 Implants only, but now that restriction is no longer in place. That same N6 sound processor easily converts to and from use of the Acoustic Component; no processor exchange is needed. This is available for use by all recipients, pediatric and adult, regardless of Nucleus implant so long as behavioral audiometric thresholds can be obtained and the recipient can provide feedback regarding sound quality.
Figure 4 shows Cochlear's portfolio of electrode arrays. A Nucleus recipient with any of these devices can now use the Acoustic Component with the N6 sound processor if they have postoperative thresholds within the fitting range and can provide feedback on the sound quality; this includes both adults and children. While we know that recipients may be inclined toward whatever they've been using, whether that be a sound processor, a speech coding strategy, etc., the benefits of combined acoustic and electric hearing are quite convincing. If you have a patient who's been implanted even for a couple of years, if they have thresholds in that fitting range, you might want to give it a try and make decisions after they've had a period of time to adjust.

Figure 4. Cochear's portfolio of electrode arrays.
Hybrid L24 Clinical Trial Outcomes Update
Now let's delve into the clinical trial data that was gathered beyond six months post-activation, the study endpoint information provided to the FDA.
Background
Although the primary endpoint was six months, we kept the study open and we continued to gather data on participants until FDA approval. During that time, the Nucleus 6 Processor was released and all trial participants were given the option to upgrade. With this processor comes advanced features such as True Wireless Connectivity, and advanced signal processing algorithms such as SmartSound iQ. Prior to this, study subjects had been using an older generation processor, the Freedom Hybrid Sound Processor. The data that we submitted to the FDA for device approval were gathered using this older Freedom Processor with minimal signal processing algorithms.
All Hybrid implant subjects upgraded to the Nucleus 6 sound processor, and 78% (39 of 50) consented to participate in the study of that upgrade. That occurred at about 3 years post-activation, depending on the individual’s surgery date.
Both professionals and recipients often ask about long-term hearing preservation; recipients ask how they will perform if they use the true Hybrid configuration versus electric alone? These data provide some answers.
Hearing Preservation - Definitions
Let's start with definitions of hearing preservation how we measure and report it. In our original discussions with the FDA, we established a couple of definitions: preservation of measurable acoustic hearing, and preservation of functional acoustic hearing. Measurable hearing suggests detection or audibility at any frequency, but it is not necessarily a measure of usability. Functional acoustic hearing was defined as a severe or better degree of hearing loss post-implantation that may provide a level of acoustic hearing to enhance electrical stimulation. Since then, we've seen functional and measurable hearing defined in different ways depending on the publication and the manufacturer.
Let's look at some of the possibilities, keeping in mind that our ultimate goal is to provide a combination of acoustic and electric hearing in order to maximize benefit, especially in challenging listening environments. It's important to measure hearing preservation consistently over time.
Reporting on Residual Hearing
The data we provided to the FDA at six months post-activation were 90% measurable and 66% functional hearing, based on a 5-frequency (125 – 1000 Hz) low-frequency pure tone average. Using these same definitions at three years, we now have 86% of the subjects who continue to maintain measurable hearing out to three years, and 60% have functional hearing. I want you to keep these numbers in mind, but I want to talk more about clinical relevance.
According to Sheffield et al. (2015), hearing less than or equal to 80 dB up to 500 Hz in the implanted ear, in combination with acoustic hearing in the non-implant ear, provides benefits for hearing in situations with diffuse background noise. Gifford and Dorman (2012) discussed how much low-frequency hearing is needed to observe an electro-acoustic benefit by reviewing previous literature. They confirmed that the addition of the acoustic fundamental frequency, around 127 - 184 Hz, was key.
In the past, Cochlear has focused on the 5-frequency, low-frequency pure tone average as a way to represent hearing preservation in Hybrid Implant patients. While this low-frequency pure tone average gives a good overview for each patient, it doesn't necessarily represent the clinical utility of preserved hearing. Very good or very poor thresholds at one or two frequencies can skew the average such that it doesn't tell the story. Let me give you an example using the 5-frequency, low frequency pure tone average using these thresholds: 45 dB HL at 125 Hz, 65 dB HL at 250 Hz, 80 dB HL at 500 Hz, and no response at 750 Hz or 1000 Hz. In Cochlear's Pivotal Study, in order to differentiate a no-response from a true auditory response, we conservatively included a no-response as a value of 132 dB HL (far beyond the output of typical audiometer). In the case that I just described, the 5-frequency, low-frequency pure tone average would then be calculated at 90.8 dB, outside of the 90 dB functional category. Honestly, it kind of makes us our own worst enemy, as with thresholds of 45, 65, and 80 at 125 - 500 Hz, this person has usable hearing and could absolutely benefit from the Acoustic Component and Hybrid Hearing. However, this person would be lumped into the non-functional category based on a 5-frequency low frequency pure tone average that's greater than 90 dB. Obviously, if you include different frequencies in that average it will change the results. If we are looking at what is functional and measurable, it can put you over an arbitrary line. We're looking at absolute values here that don't always represent clinical relevance.
Let's look at some other possibilities. As I mentioned earlier, we reported a 66% functional hearing preservation rate at six months, and 60% at three years. But did you know that 78% of subjects use the Acoustic Component at three years, and that number remains stable after 12 months? We didn't require use of the Acoustic Component based on thresholds, rather, clinicians were given the specs of the Acoustic Component and encouraged to fit it if possible. Ultimately, subjective impression and objective validation were considered and decisions were made accordingly.
So another way to look at hearing preservation or useful residual hearing might be to see how many use the Acoustic Component. In this case it was 78% after three years of implant use. Finally, it’s important to consider those subjects have residual hearing good enough that they don't even need the Acoustic Component. We've certainly seen people who have more than enough hearing in the low frequencies to get the benefits of natural acoustic hearing without wearing an Acoustic Component. We're looking for all of our patients to get the benefits of electro-acoustic stimulation in any way possible.
L24 Residual Hearing at 3 Years
In our study, we looked at pre-op thresholds as compared to median thresholds 3-years post activation. Given that Nucleus Acoustic Component amplifies up to 90 dB out to 2200 Hz, we found that median thresholds at three years post-activation support the use of electro-acoustic by the majority of subjects. Those individuals can then get electrical stimulation from the cochlear implant in the mid to high frequencies in order to complete the picture for optimal speech understanding.
After years of studying the Hybrid implant and outcomes, we have learned that how much your hearing changes after implantation doesn't necessarily matter. Where you land is what's important for determining clinical benefit. Research confirms that any low frequency hearing preservation, especially at and below 500 Hz, has the potential to give an individual all of the benefits of EAS including natural sound quality and good speech understanding, especially in noise. These are very exciting results.
Speech Perception Outcomes Over Time
CNC in Quiet at 60 dBA. To look at speech perception, we used unilateral CNC word scores in the implanted ear. We used CNC data because CNC words were used for the candidacy measure in the data that we provided to the FDA that lead to approval. At six months post-op, study subjects scored an average of 65.4%. The trend showed continued improvement at 12 months with a mean score of 74.4% and those results remained stable out to three years. Improvements seen at each post-operative interval when compared to pre-op were statistically significant, while any differences between post-operative intervals were not significant.
Sentence Materials. Our goal as clinicians when we test our patients is to figure out how they're doing now so we can see where we need to go next in order to provide continued improvement in performance and benefits. Over the years, we've seen changes in our cochlear implant candidate population. You may remember when CID Everyday Sentences were used as part of the MAC battery which also included a Four-Choice Spondee test and tests of medial vowels and medial consonants, for example. This was at a time when candidates for cochlear implants had little, if any, hearing preoperatively and we were hoping for even small improvements in basic speech recognition abilities. Over time, things have evolved in terms of the candidates, the indications, and the technology. As a result, there has been a need to shift to more difficult tests in order to avoid ceiling effects. In Cochlear's earliest clinical trials with Hybrid Implants in 1999 we used CUNY Sentences, but we found that subjects quickly topped out so this was not a good way for us to measure performance changes over time. Around the same time, members of the audiologic and medical communities got together to establish a Minimum Speech Test Battery (MSTB) in attempt to standardize test procedures across clinics. Part of this MSTB was a move from CUNYs to HINT Sentences in Noise.
In 2004, Spahr and Dorman developed the AzBio Sentences. Use of this measure in the presence of competing background noise has since become the standard in a revised MSTB from 2011. They are also what was used in our Hybrid L24 Clinical Trial in attempt to avoid ceiling effects.
In 2006, Dorman and Spahr compared performance on various speech recognition tests, in addition to other demographic factors, in patients with average and above average performance. While the signal-to-noise ratio they used is different than that used in current Hybrid studies, their data show relative differences. They found that performance on AzBios is generally not as good as that on the CUNY Sentences, given that it's a harder test. In another publication in Cochlear Implants International (2016) from a group of researchers out of Australia, the authors examined ceiling effects on various sentence tests and found that in their group of post-lingually deafened adults, CUNY test scores skewed to the right with a large majority of subjects scoring above 90%. This suggests that ceiling effects are likely to be reached and a more difficult test is needed to measure true change in performance from pre to post-operative evaluations.
If you listen to CUNY Sentences and AzBio Sentences, you can compare the materials and see for yourself how they might influence the results. There is a significant difference in the rate of speech - the CUNY Sentences are presented at a slower rate, and AzBios use a rate similar to conversational speech. Also, CUNY uses a male talker whereas AzBios uses a combination of males and females. You may notice other differences as well. Overall an easier test may be used to help make your patient feel more confident but a more challenging test will be better able to help us measure performance changes over time. Sometimes, using a combination of materials could be helpful.
In our Hybrid L24 study, we measured performance on AzBio Sentences in noise at a +5 dB signal to noise ration (SNR). The pre-op score for the 39 L24 subjects who upgraded to the Nucleus 6 Processor and participated in this amendment study was just under 17%. On the day that these subjects upgraded to the Nucleus 6 Processor, the average score jumped to over 70% correct with the Nucleus 6. Recall the relative stability of audiometric thresholds and CNC word recognition when comparing 12 months to three years post-op. So why the jump in performance for sentence testing in noise? This is likely due to SmartSound iQ, the suite of advanced signal processing algorithms in the Nucleus 6 Processor. It includes automatic directionality and noise algorithms that are specifically designed to improve understanding in the most challenging environments. We were very happy to see that our Hybrid recipients could benefit.
Power of the Nucleus 6
Figure 5 shows the signal processing algorithms that are available in the Nucleus 6. The algorithms that are available for the acoustic part of the signal are on the left side of the figure, and the algorithms that are available on the electric part of the signal are on the right. I want to remind you that data from the FDA trial were collected with an older generation sound processor, the Freedom Hybrid Sound Processor. While the recipients could use ASC, ADRO, and BEAM In their everyday lives, when data was being collected, all input processing capabilities were turned off. Additionally, when we tested electric-only modes throughout the study, subjects were using their abbreviated electric MAP, perhaps with some electrodes deactivated, and they weren't given a full-frequency electric MAP for testing purposes. Hybrid recipients continue to benefit from updates in technology, and the improvement in performance may be significant, as we discussed with the AzBio Sentences in our study.

Figure 5. Signal processing algorithms available in the Nucleus 6 Sound Processor.
You might be interested to see how patients do using electric-only stimulation. As clinicians, we need to feel comfortable recommending technology to our patients, and we don't want to second-guess how they might have done with a different option. A frequent question we receive from clinicians who are new to using Hybrid is, "Would my patient be better off with a regular cochlear implant?" To try and answer that question, let's review some data.
Speech Perception in Noise
We tested AzBio Sentence scores in noise (+5 dB SNR) for the 39 subjects as part of this upgrade study. These data were collected preoperatively with the best fit hearing aids, and then at three years post-activation with subjects separated out by those who use Hybrid Hearing, those who used electric only stimulation, and then the whole group combined together.
For the electric-only group, there was significant improvement from pre-op to three years post-activation. With the subjects using Hybrid hearing, there was further improvement in post-operative scores compared to the electric-only group. It's important to note that these results were obtained in the unilateral condition. In the bilateral condition, we consistently saw about a 6 - 10% increase on every test over the best unilateral condition.
What happens to people who have a traditional cochlear implant? Let's answer that question. We looked at average performance on the same test in the same condition in a group of 40 traditional cochlear implant recipients who were upgraded to the Nucleus 6 Processor in a parallel study. We compared their results to the Hybrid subjects in this study.
Average performance in this long electrode array group was on par with the electric-only performance for the Hybrid subjects. So, this would suggest that your Hybrid patients in the electric-only condition have as much potential as traditional cochlear implant recipients, and far better than what they were getting preoperatively. For those who use the combined electric and acoustic signals, performance is even better than what we see with the average traditional cochlear implant.
Further Post-Approval Studies
Cochlear is currently working on two separate post-approval studies. These are considered Phase 3 studies and they are also regulated by the FDA with the goal of collect long-term data on safety and effectiveness. A post-approval study involves a bigger population, more subjects, more centers, and a greater variety of clinic environments.
The two post-approval studies that Cochlear has underway are separate but related. The first study is an extended follow-up study on our original Hybrid L24 subjects. Those same subjects whose 6 month data we presented to the FDA, and whose data we just reviewed at three years, have consented to continue to participate in study evaluations out to five years post activation. We have five year data now on 32 subjects and we're working on compiling those data for presentation and publication. Keep an eye out for updates at key meetings, on Cochlear's blog and in the literature in the coming months.
Cochlear's second active post-approval study is also a five year study, but in a newly implanted group of people. We're currently working with 19 implant centers across the US who are enrolling and implanting Hybrid L24 candidates with the intention of following them for five years. These data will add to our current body of data. Data collection and analysis is ongoing and in the end, we will have a large number of subjects and a lot of data to pool and analyze from the various studies.
Programming Considerations
I will now give you an introduction to the Custom Sound software for those of you who are not familiar with it, and review some recent changes for those of you who may already be programming the Hybrid.
I want to take a moment to highlight our ongoing alliance with GN ReSound. We initially collaborated with ReSound on the development of the industry's first True Wireless System, which serves to improve recipients’ access to sound. We've strengthened that relationship to include a collaboration centered around optimizing bimodal fittings, which is an important part of our expanded indication. I know of many study sites in the newly implanted post-approval study who are taking advantage of fitting a ReSound hearing aid in the contralateral ear and using the True Wireless accessories with great results.
We have learned a lot over the last couple of years with regards to programming this patient population. We will review what we know today on how we should optimize fittings.
According to the literature to date, we believe that you should fit what you can acoustically to the point where the patient is demonstrating and perceives benefit. The fitting range of the N6 Acoustic component is for frequencies with thresholds less than 90 dB, although some recent presentations at American Cochlear Implant Alliance conference noted ranges for electro-acoustic fittings between 70 and 85 dB as alternatives based on individual clinical experience. Patients with normal to mild post-operative thresholds may not even need to be fit with the Acoustic Component but rather, allowing their open, natural ear to provide the low-frequency information. Conversely, recipients in the lower end of the fitting range need to be thoughtfully managed. We've seen in recent publications such as Dillon (2014) that verification is important to optimize outcomes. It is very important to verify that the information provided electrically connects or overlaps with the information provided acoustically to ensure that there are no gaps in audibility. This is where functional gain measures may play a role. Modifications to our fitting guidelines are based on current research being conducted on Hybrid Hearing recipients as well as the review of ongoing clinical cases in the field.
I'll share with you an example of a clinical case that demonstrates how Custom Sound applies the fitting formula and why changes may need to be made. When fitting a Hybrid Hearing system, Custom Sound uses the audiogram to determine which frequencies receive an acoustic signal, which receive an electric signal, and which receive both (referred to as overlap). Custom Sound automatically provides a MAP with a minimal amount of overlap between acoustic and electric signals. The lower electric frequency boundary can be modified within Custom Sound if necessary.
What's Changed in Custom Sound 4.4?
In Custom Sound 4.4, we have revised the low-frequency cut-off for electric stimulation in Hybrid mode. The cut-off for acoustic amplification hasn't changed, which also means that candidacy for the Hybrid hasn't changed. As we've previously advised via our Hybrid Desk Reference and now implemented in the software automatically, once the entered audiogram thresholds become greater than 70 dB (measured from left to right up to 2000 Hz), the electrical channels are enabled. Since the Acoustic Component will be active for thresholds up to 2000 Hz that are less than or equal to 90 dB, this will result in more overlap between electric and acoustic for some audiograms. This change in where the cut-off is set doesn't change existing Hybrid mode MAPs. However, if you create a new Hybrid mode MAP for an existing recipient, the new calculation is applied. This is summarized in Figure 6.
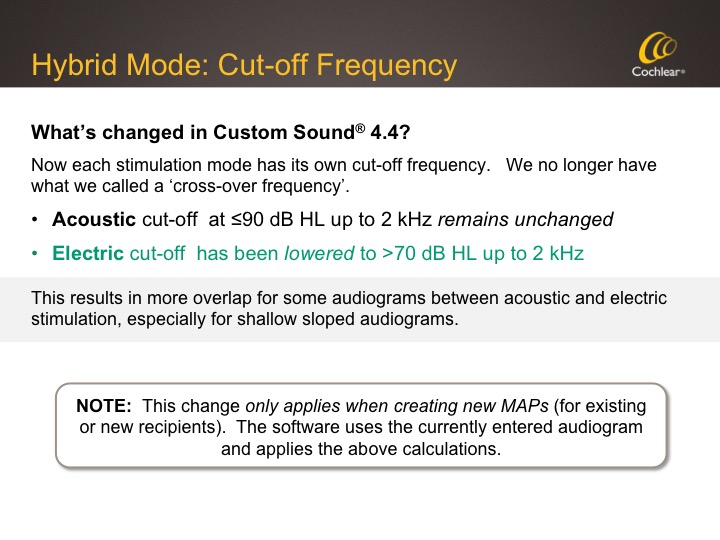
Figure 6. Hybrid Mode: Cut-off frequency.
Key Takeaways
In terms of key takeaways, it's important first to verify what can be fit acoustically through real ear measures. Real ear measures are always the gold standard; if real ear measures are not possible, verify by sound field thresholds. If target can't be met or there's not good audibility for that frequency after making gain adjustments, then disable the acoustic channel and provide an electric signal at that frequency. Remember, there's no problem with providing electric stimulation; that is why these people were implanted.
A second key takeaway is that the amount of overlap between the acoustic and electric signal may vary. While there's ongoing research investigating the amount of overlap to provide a recipient, currently there's not a clear consensus on how much overlap to apply. Therefore, the extent may vary depending on the amount of residual hearing as well as on individual listening preferences. Consider providing additional MAPs with a lower CI cut-off frequency.
Figure 7 shows the specs for the Acoustic Component so that you can see the output and also note that the fitting range is out to 2200 Hz.
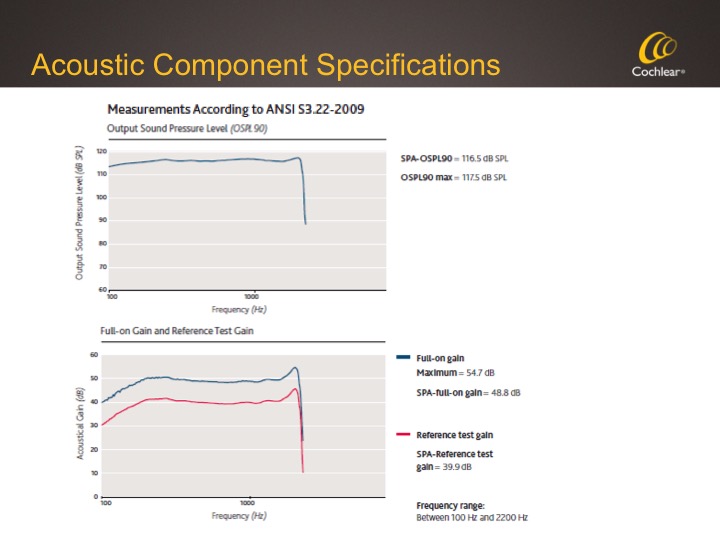
Figure 7. Acoustic Component specifications.
Let's look at how the software applies electric and acoustic stimulation from a practical standpoint. When you're programming in Custom Sound, the software automatically checks the thresholds that you've entered from left to right. It checks where the threshold crosses 90 dB HL, up to and including 2000 Hz. It uses that information to set the acoustic cut-off frequency band; acoustic stimulation will be applied up to the frequency from left to right that crosses 90 dB HL.
At the same time, the software checks where the threshold crosses 70 dB HL from left to right, in order to determine the first electrical frequency band for the MAP. This can result in greater overlap of electro-acoustic stimulation. What we know is most important is to not have a gap between electric and acoustic, and this will help to avoid that gap.
The audiogram in the upper left of Figure 8 has thresholds of 70 dB HL at 500 Hz and 100 dB HL at 750 Hz. Since the 90 dB level is crossed between 500 Hz and 750 Hz, acoustic stimulation will extend up to 685 Hz. Since hearing is poorer than 70 dB HL above 500 Hz, the electrical stimulation is prescribed starting at 563 hertz. This results in an overlap of 122 Hz. As always, you'll want to verify the Acoustic Component with real ear measures and make any adjustments based on your results. You can choose to make additional overlap MAPs or make other adjustments based on your real ear measures as well as on your patient's speech perception outcomes after some use time.
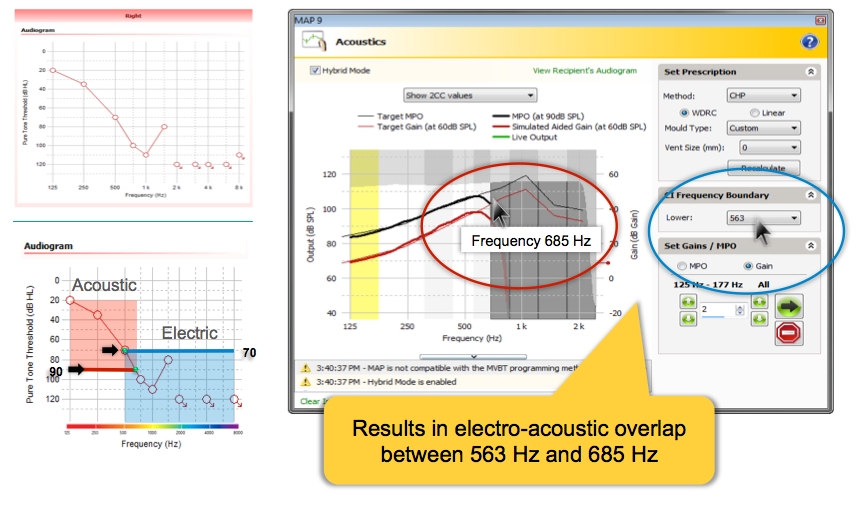
Figure 8. Electrico-Acoustic crossover ranges. /38
Steps in Programming
Now we will review the steps to program a new user with Hybrid Hearing.
Step 1 - Create a new recipient. First, you'll create a new recipient and then click the Acoustics tab. In the Acoustics tab, enter the audiogram, including inter-octave frequencies, and note where thresholds are equal to or better than 70 dB HL.
Step 2 - Measure impedances. The next step, as always, is to measure impedances.
Step 3 - Create a new MAP. Custom Sound 4.4 has a default pulse width (PW) of 37. Check the Hybrid Mode box on the far right.
Step 4 - Remove the coil from the head. Click on the Acoustic icon in the toolbar to open up the Acoustic Screen. Select the fitting prescription, the compression, and the vent size. Note that you can now select the type of mold, whether it's unknown, a custom ear mold, a Power Dome, or a Plus Dome in the fitting software. When you select a Power Dome or a Plus Dome, additional gain will automatically be applied to the target gain.
Step 5 - Go live with acoustic stimulation only in the Acoustic screen. Complete real ear measures to verify that the output at each frequency matches the slope and shape of the target curve and doesn't exceed the UCL for the recipient. You can make any necessary adjustments to the gain and MPO for all channels, as required, using the up and down arrows under All or on a specific identified channel. Make sure you take into account the subjective feedback of the recipient, and if the desired acoustic gain can't be met in a channel, you may need to enable an adjacent channel to determine if additional gain is made available to meet the target. And if not, you may need to lower the CI frequency boundary and disable any acoustic channels that didn't meet target. Next, stop the acoustic stimulation.
Step 6 - Measure electrical stimulation. Place the coil back on the patient's head. Measure T levels using the streamlined programming method, go live, set C levels using a global shift in live voice with both acoustic and electric stimulation. Make adjustments so the sound is comfortably loud to the recipient, stop the stimulation, and then sweep at C level in small groups to loudness balance.
By default, electrodes 1 - 3 are disabled in the software. That is a conservative method that we've applied to make sure that all of the electrodes are actually in the cochlea. There's no need to leave those channels off if the electrodes are in the cochlea. We recommend that you turn each of them on one at a time, and test them out to make sure that they're comfortable for the recipient.
Acoustics Panel Option
Now, in Custom Sound 4.4 it's easy to make quick loudness comparisons between Hybrid mode and full electrical mode. You have the ability to do that in the different modes in which the patient is listening; you can let them listen in Hybrid mode and then also with just the Acoustic Component.
Once you've optimized the Acoustic Component, consider saving the Hybrid MAP. If you intend to uncheck the Hybrid MAP box in the software, doing so will result in a full electric MAP with the default frequency allocation (188 Hz - 7938 Hz). I want to reiterate that point: you have a Hybrid MAP to start, and if you uncheck Hybrid MAP, the software will automatically reallocate to a full frequency electric MAP in order to be sure that someone who's not using Hybrid stimulation has access to all of the speech frequencies. This is a change, as previous versions of Custom Sound would not reallocate the frequency boundaries when Hybrid MAP was unchecked, and could have resulted in a limited frequency electric MAP when full frequency was actually needed. Now, the assumption is that if you disable Hybrid mode, you're doing so because you want all electric stimulation across the full range of 188 to 7938 Hz. This may be a useful way to allow for comparisons for recipients for are undecided as to the use of the Acoustic Component.
Returning to the Hybrid MAP by rechecking the box will revert the Acoustic Component to the default settings. For that reason, we suggest that you save your MAP prior to checking or unchecking the box in order to preserve any changes that you may have made to the prescription.
You also now have the ability to check the Mute Acoustic box. When you check the Mute Acoustic box, you will no longer see the Live Output feedback green line. Checking the Mute Acoustic box doesn't affect the frequency allocation of the MAP, it simply mutes the Acoustic Component to let the recipient listen to only the electrical stimulation. When you uncheck the Mute Acoustic box, you get the Live Output green line back, which tells you that the Acoustic Component is live and the recipient is listening in electro-acoustic or Hybrid mode again.
Summary of Quick Loudness Checks. In order to listen to full electro-acoustic stimulation in Hybrid mode, make sure that you have a Hybrid MAP selected. In order to listen to the electric component only, check the Mute Acoustic box to provide only electric stimulation. If you want to only listen to the Acoustic Component, lift the coil off the head. And, if you want to move someone to a full-frequency, cochlear implant-only, electric MAP, uncheck the Hybrid MAP.
Final Programming Steps
In order to finalize programming, check your batter suitability and personalize the MAPs by adding SmartSound iQ input processing, tones, alerts, and telecoil as needed. Save the MAPS, write to the processor and close the session. We recommend Program 1 as the primary MAP with SCAN. The outcomes with this automatic scene analysis algorithm are remarkable. In program two, and program three and four, you can try other defaults or a patient preferred MAP. Finally, write the MAPs to the processor and finalize the programming session, and then verify audibility.
Figure 9 shows a MAP that was verified in sound field and the Acoustic Component is giving thresholds that are substandard, while the electric portion is giving good information. If you see this on sound field audiogram, we recommend raising the gain on the Acoustic Component, or if that's not possible, then consider adding additional electric stimulation to adjacent channels so that you get a good sound field audiogram.
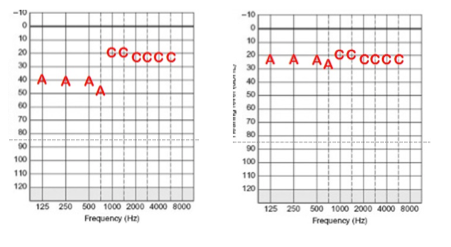
Figure 9. Substandard Acoustic Component thresholds (left) as compared to a good sound field audiogram (right).
Keep in mind that the default setting for the sound processor is to turn off automatically after two minutes when the coil is not on the head. If you are going to be testing acoustic alone in the booth and taking the coil off, you might want to deactivate the auto processor off in Custom Sound to make sure that the sound processor doesn't turn off automatically.
Summary
The Hybrid indication is continuing to gain momentum. We're seeing people come in to our offices for this technology who are struggling with hearing aids and need another option. Programming of both the electric and acoustic portions can be completed with the Custom Sound software. While the overlap between acoustic and electric stimulation will vary by patient, there is overlap guaranteed now with the default software settings. The combination of the Hybrid L24 and the Nucleus 6 Processor provides outcomes far beyond what we've seen in the past: 95% of these subjects perform the same or better on AzBios in Noise (+5 dB SNR) and there are improvements on CNC words. Additionally, there is stability of hearing thresholds out to three years post activation. Electric-only patients continue to perform comparably to the average traditional cochlear implant recipient If someone needs to use an electric only MAP, their potential is just as high as what it would have been if they had a long electrode array in the data that we've seen. Hybrid Hearing continues to provide superior outcomes to traditional cochlear implants. Hybrid Hearing is now FDA-approved and available for all recipients with Nucleus implants. To summarize candidacy criteria, the indications are as follows:
- Ear to be implanted: Aided CNC word score between 10% and 60% correct, inclusively.
- Contralateral Ear: Aided CNC word score better than ear to be implanted but less than 80% correct.
- Audiometric: Severe to profound high frequency sensorineural hearing loss, bilaterally.
- Adults, age 18 years and older.
For more information, please visit www.cochlear.com/us
References

Citation
Grant, G. (2017, June). Hybrid L24 study update: where are they now? AudiologyOnline, Article 19713. Retrieved from www.audiologyonline.com


