Learning Outcomes
After this course learners will be able to:
- Describe the role of hearing loss in aging and public health.
- Describe the relationship between hearing and functional outcomes based on past and current epidemiologic studies and clinical trials.
- Describe the role that hearing healthcare providers have in addressing hearing loss in adults.
Introduction and Overview
Thank you for your participation in this presentation today. I am an ENT Otologic Surgeon at Johns Hopkins, but I spend the majority of my time doing public health research. I direct a research center at the School of Public Health called the Cochlear Center for Hearing and Public Health, which is dedicated to thinking about and addressing the impact of hearing loss on aging and public health. Today, I'm going to take you through some of the research that we've been conducting, as well as share some of the broader ideas as to why we now think that hearing loss may be a critical component of aging, particularly from a public health perspective.
When people think about public health, they may not typically think about adult hearing loss. As we begin thinking about the rationale that frames much of the work we do here at Hopkins, you may ask the question "Why hearing and public health?" According to 2015 data from the United Nations, there are roughly one billion older adults in the world aged 60 and older. Over the next 30 to 35 years, it is projected that in many parts of the world, the number of individuals who are age 60 and above will double. In the next few years, for the first time ever, we'll have more older adults than young children worldwide.
Eras of Public Health
Thinking about this demographic change from the broad public health point of view, the reason why this population increase has occurred is because we have progressed through a few different eras of public health. About 100 years ago, for the first time in all 200,000 years of human evolution, we essentially conquered infectious disease. Tuberculosis and dysentery used to be the leading killers of all of mankind, tuberculosis being the single etiological agent that has killed the most number of humans in all of history. In the last one hundred years, we began to control and conquer these diseases through immunizations, clean water, and antibiotics. That was the first era of public health, which is still ongoing, but essentially we've moved through the error rate to the present day.
Throughout the 20th Century, the leading cause of death in humans has been from chronic diseases, such as heart disease, stroke, and diabetes. This is what we consider to be the second era of public health. I wouldn't say we're out of the woods yet by any means, but we've clearly made a lot of progress. It is these first two eras of public health that we've moved through on an enormous scale. This explains why we're seeing populations increase, as people are living longer than ever.
Now, we're rapidly entering what people consider to be the third era of public health, which involves the challenge of dealing with aging processes themselves. I use the plural word "processes" because there is not one, single aging process, but many. These aging processes are set into motion as we get to be age 50 and over. A major challenge of this third era of public health is how do we begin confronting all these older adults? It's not enough to prevent infectious diseases and treat chronic diseases. What do we do as a society to allow these older adults to live maximally and fully as they go forward?
Health and Policy Strategies Needed for Healthy Aging
Many schools of public health and gerontology around the world have studied this topic in depth. The big picture question is: What are the health and policy strategies that are needed for optimal healthy aging?
Typically, the first strategy that people study and employ is how to prevent and delay disease. This entails what we call compression of morbidity. The notion of compressing morbidity means that rather than someone having a disease their entire life, the aim is to compress it so that it occurs at the very end of life. Equally important is the effective treatment of disease. Nowadays, a main topic of interest in gerontology and geriatrics is the management of multimorbidity. Usually, older adults in their 60s, 70s, and 80s don't just have one ailment, such as diabetes. They also have diabetes and hypertension and arthritis. How do you manage all of these conditions together to optimally allow someone to thrive? Additionally, there has been a lot of study on lifestyle factors, such as diet, activity, and social engagement. Furthermore, a lot of research has been conducted related to the biological processes underlying aging. Are there certain therapeutic drugs that would be used to minimize the effects of aging over time?
Finally, one increasingly popular strategy that can be implemented to promote healthy aging is by optimizing sensory function. There have been a lot of converts to this conceptual model of thinking. Looking at aging from the perspective of public health, as physicians, as clinicians, as researchers -- we all care about the brain. The brain is the most important organ in the body. It's the only organ that can't be replaced. A person can undergo a lung or liver transplant, but you can't replace someone's brain.
In the arena of medicine and public health, we commonly study how the brain is related to thinking and memory abilities, to our physical function, and to our mental/social functioning. Increasingly, people have been looking at how diseases and other risk factors affect our neural pathways. When we evaluate our methods of how research is dictated, we often forget about our senses. We take for granted that our brain is locked in our skull and the only way our brains are able to perceive the world around us is through our senses. As we think about healthy aging, we are increasingly looking at the senses. If a person is not dying of heart disease, and we have controlled the risk factors we can control, in order to live a full, active life as an older adult, we require the use of our senses. Ultimately, aspects of cognitive, physical and social functioning are completely dictated and mediated by our senses. If we don't have our senses, we aren't fully interacting and engaging with the people and the world around us. In many ways, our senses underpin everything we care about within aging and public health, once we get beyond controlling and preventing diseases.
Prevalence of Hearing Loss by Age
Across the lifespan, the prevalence of hearing loss (defined as a better-ear pure tone average of 0.5 - 4 kHz tones > 25 dB) almost doubles every ten years. In the United States, nearly two out of every three adults over the age of 70 has a meaningful hearing impairment (Lin, Niparko, & Ferrucci, 2011).
The premise that drives a lot of the work that I do is this public health approach to thinking about hearing. It is the fundamental belief that our ability to hear and engage with the people and environment around us is absolutely critical to our health and well being. As we get into later stages of life, if we've controlled disease as best we can, that is the time when these senses become extremely important.
If we think about hearing loss and aging from a broad, public health perspective, there are some basic questions to ask to determine whether this belief is true or not.
- Are there meaningful consequences for people with hearing loss compared to people with normal hearing? The way we answer this question is with epidemiological large studies of older adults.
- What is the impact of treating hearing loss in older adults? Do our interventions make a difference on broader outcomes?
- How can hearing loss be effectively addressed in society?
Consequences of Hearing Loss for Older Adults
For the duration of the presentation, we will look at some of the progress that has been made in this area, particularly from some of the work we're doing at Hopkins, as well as things that need to be done going forward. As stated earlier, although I am an otologic surgeon, the research I do comes from the perspective of public health. This concept of healthy aging is what drives my research and my academic interests, not hearing loss in and of itself.
Healthy aging is a concept with which we're all likely familiar and we would recognize it when we see it. For example, a man who lives in the U.K. named Fauja Singh took up running in his 80s and ran marathons up until he turned 100 years old. In addition, we can look around us and see older adults who lead very active lives and as they age, they remain socially engaged with family and friends. Those are dynamic examples of healthy aging. In contrast, there are people with mobility impairments or dementia living in nursing homes, which would not be considered as ideal healthy aging.
From a public health research perspective, we have defined several different domains of healthy aging:
- Maintaining cognitive vitality and avoiding dementia
- Avoiding injury
- Maintaining physical mobility and activity
- Positive mood and mental health
- Keeping socially engaged
In addition to these facets of healthy aging, beginning in around 2010, we began asking the question of whether hearing loss has a meaningful impact on the broader outcomes related to healthy aging.
For the purposes of today's presentation, we will focus on the condition of dementia. With the increasing aging population in the U.S., older adults are at risk of developing cognitive decline severe enough to become dementia at some point in their lives. By 2030, an estimated 8.4 million adults aged 65 and older will have Alzheimer's Disease. It is projected that dementia rates in the United States will triple between now and 2050. To put that in perspective, by 2050, an estimated one out of 30 living Americans will have dementia. Not just one in 30 older Americans -- one in 30 of every living American will have dementia (Hebert, Weuve, Scherr, & Evans, 2013). Again, that is because of the number of older adults who are beginning to dominate population demographics. Despite that statistic, to this present day, no therapeutic agents or treatments exist that can prevent or even delay dementia. However, in the last five years, the National Institute on Aging has seen its budget triple from one billion to over $3 billion. That $2 billion increase is purely because of the need for dementia research.
Mechanisms Linking Hearing Loss to Cognitive Decline/Dementia
How is hearing loss related to cognitive decline and dementia? Could it be that they are related through some type of common pathological process? For example, as you get older, you're more likely to have hearing loss and you're more likely to have cognitive decline. Perhaps cognition is affected by conditions like diabetes, hypertension, or smoking (which affects the small blood vessels that go to the brain and the ear). Maybe it is just a common pathologic process leading to both. The real question is whether there are, in fact, mechanisms that link hearing loss to cognitive impairment and dementia? Increasingly, we believe there are.
Cognitive load. One mechanism which has been hypothesized to link hearing loss and cognitive decline is the idea of cognitive load. In other words, when you can't hear well, the ear is constantly sending a garbled auditory signal to the brain. Does the brain constantly have to work harder to process that poor signal? Is the brain reallocating resources, per se, to constantly dealing with that garbled auditory signal and does it come at the expense of other systems?
In 1973, Danny Kahneman put forth his model of cognitive resource capacity (Kahnerman, 1973). Cognitive resource capacity is the idea that we have a pool of cognitive resources for thinking, planning, memory. We now know that over time with aging, we can lose some of these resources from things such as synaptic loss of the brain. Increasingly, we are beginning to understand that a hearing loss likely taxes the system as well. The brain is constantly having to re-allocate resources to help with deciphering and decoding that much more garbled auditory signal.
The sensory system, particularly the hearing system, is fundamentally unique. It's a system that is always on. Even in the middle of the night, we are still processing sound. We are constantly processing auditory stimuli to learn about the auditory environment. It comes from an evolutionary perspective. We can't turn this system off.
Jonathan Peale and his team at Washington University in St. Louis conducted some studies using functional MRI images. They looked at patterns of brain activation in people with poor hearing (mild to moderate hearing loss) versus same-aged peers with good hearing. Consistently, MRI images reveal that people with poor hearing have reduced activation of the primary auditory cortex (the part of the brain critical for sound processing). That makes sense. If you have an impoverished auditory signal from the ears because of hearing loss, you get reduced language-driven activity in primary auditory pathways. Interestingly, in these same people with hearing loss, we also observe what we believe to be increased compensatory language-driven activity in the prefrontal cortical areas of the brain. The prefrontal cortex part of the brain is typically not needed for sound processing, and yet we are seeing an activation of this part of the brain in people with even a mild to moderate hearing loss (as compared to normal hearing individuals). In other words, different regions of the brain are being recruited for auditory and language stimuli in order to compensate for a reduced auditory signal, and the brain needs to expend more effort to decode the signal. This is one pathway through which hearing loss could directly impact cognitive decline and dementia.
Brain structure/function. A second, related pathway connecting hearing loss with cognitive decline is the idea that hearing loss in and of itself may lead to changes in terms of brain structure and, as a result, brain function. This finding is based on studies conducted on large groups of older adults with hearing loss over many years using brain MRI scans. In several different studies, they found that people with hearing loss have faster rates of brain atrophy. Again, we think that's because of the impoverished auditory signal which leads to cascading effects on brain structure and hence, brain function (Lin & Albert, 2014). The structural integrity of the brain dictates its function. The two most important conditions that can damage the brain over time are microvascular disease (i.e., small vessel disease from high blood pressure, diabetes, etc.) as well as the more classic Alzheimer's neuropathology. Not coincidentally, these are the two major causes of dementia in late life.
Social isolation. The third mechanism which has been hypothesized is the idea that for some people, hearing loss can lead to social isolation problems. In the field of gerontology, studies have repeatedly shown that social isolation is arguably one of the biggest predictors of morbidity and mortality in older adults. We see consistently that social isolation relates to poor outcomes through several different pathways. Health and behavioral pathways are affected by things such as adherence to medical treatments, as well as smoking, diet, and exercise. In addition, psychological pathways are impacted by a person's self-esteem, self-efficacy, sense of well-being and the ability to cope. Recently, however, one of the more compelling theories is the idea that social isolation or loneliness is directly linked to physiologic changes in the body, which precipitates adverse events.
John Cacioppo and colleagues conducted research in 2007 and 2011 comparing people who were socially isolated with those who were socially integrated. Consistently, the research suggested that socially isolated people had an increased upregulation of pro-inflammatory genes and increased inflammation, which causes a lot of adverse events and aging processes in the body. This research directly provided the link showing how loneliness leads to adverse events in older adults in later life. This finding allows us to understand how hearing loss over and above any type of common pathology is linked to adverse events like cognitive impairment and dementia, at least from a theoretical construct (Cole et al., 2007; Cole, Hawkley, Arevalo, & Cacioppo 2011).
Studying Hearing Loss and Cognition
Next, in order to determine if this finding is true from a public health perspective, we conduct epidemiologic studies. Epidemiologic studies are very different from clinical research. As defined by the National Center for Biotechnology Information (a branch of the National Institute of Health), epidemiology is the study of the distribution of diseases and other health-related conditions in populations, and the application of this study to control health problems. In an epidemiologic study, large samples of adults are recruited from the community. These are people who aren't sick already or have illnesses, but people who are just living their normal lives. An epidemiologic study is what first determined the link between smoking and lung cancer years ago.
Over the last several years, we've worked with many large epidemiologic datasets with which we have a lot of collaborations. These include:
- NHANES: National Health and Nutritional Examination Surveys. This is a large, cross-sectional representative sample of the U.S. population (~3000-4000).
- BLSA: Baltimore Longitudinal Study of Aging at the NIH. This is an ongoing prospective study of over 1000 older adults since 1958. This study is funded by the National Institute on Aging (NIA) and takes place in Baltimore (where the NIA campus is based).
- HealthABC: Health, Aging and Body Composition Study. This is a prospective, population-based study of ~3000 adults aged 70 years and older.
- ARIC: Atherosclerosis Risk in Communities Study. This is a prospective, population-based study of ~16,000 adults followed since 1977.
These are all large studies of community-based older adults. These are not clinical samples. These are people living in the community. In many cases, fortunately, they have had their hearing measured objectively with pure tone audiometry and the other outcomes measured as well.
Measuring Cognition
Before I show you some of these research findings, I wanted to define a little more what cognition is. Cognition is intuitive for many people. It's the idea of our thinking and memory. The way we measure cognition from an epidemiologic and public health standpoint is by conducting tests that assess the different domains of cognition. These cognitive domains are:
- Memory (Free and cued selective reminding test, or FCSRT)
- Executive function (if you're given two different things to do at once, can you pay attention to one thing and ignore the other)
- Trail Making B
- Stroop Mixed
- Digit Symbol Substitution
- Psychomotor/processing speed
- Verbal function and language
It is important to note that these tests are not dependent on hearing. These are non-auditory tests. For the purposes of today's presentation, I will highlight the tests of executive function, beginning with Trail Making B. In this test, participants are given a big sheet of paper with numbers and letters on it (Figure 1). They are given a pencil and asked to connect one to A, two to B, three to C, etc. They can go back and forth, juggling between two different strings of information.
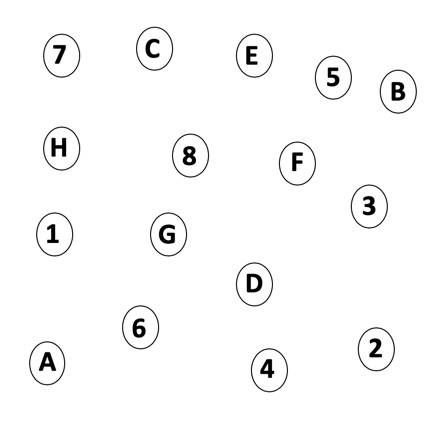
Figure 1. Trail Making B.
Another commonly used, non-auditory measure of cognition and executive function is called Stroop Mixed (Figure 2). In this test, the subject has to name the ink color, not read the word. If you try it, you will find that it is fairly challenging because reading is a completely automatic task, whereas, naming a color takes more cognitive effort.

Figure 2. Stroop Mixed.
Lastly, the Digit Symbol Substitution Test (DSS) is another commonly used measure executive function (Figure 3). The subject is given a sheet of paper with a string of numbers and a symbol underneath each number, like a code. The subject needs to complete the worksheet and fill in the code as quickly as they can. Also a non-auditory measure of cognition, the DSS test targets working memory and executive function.
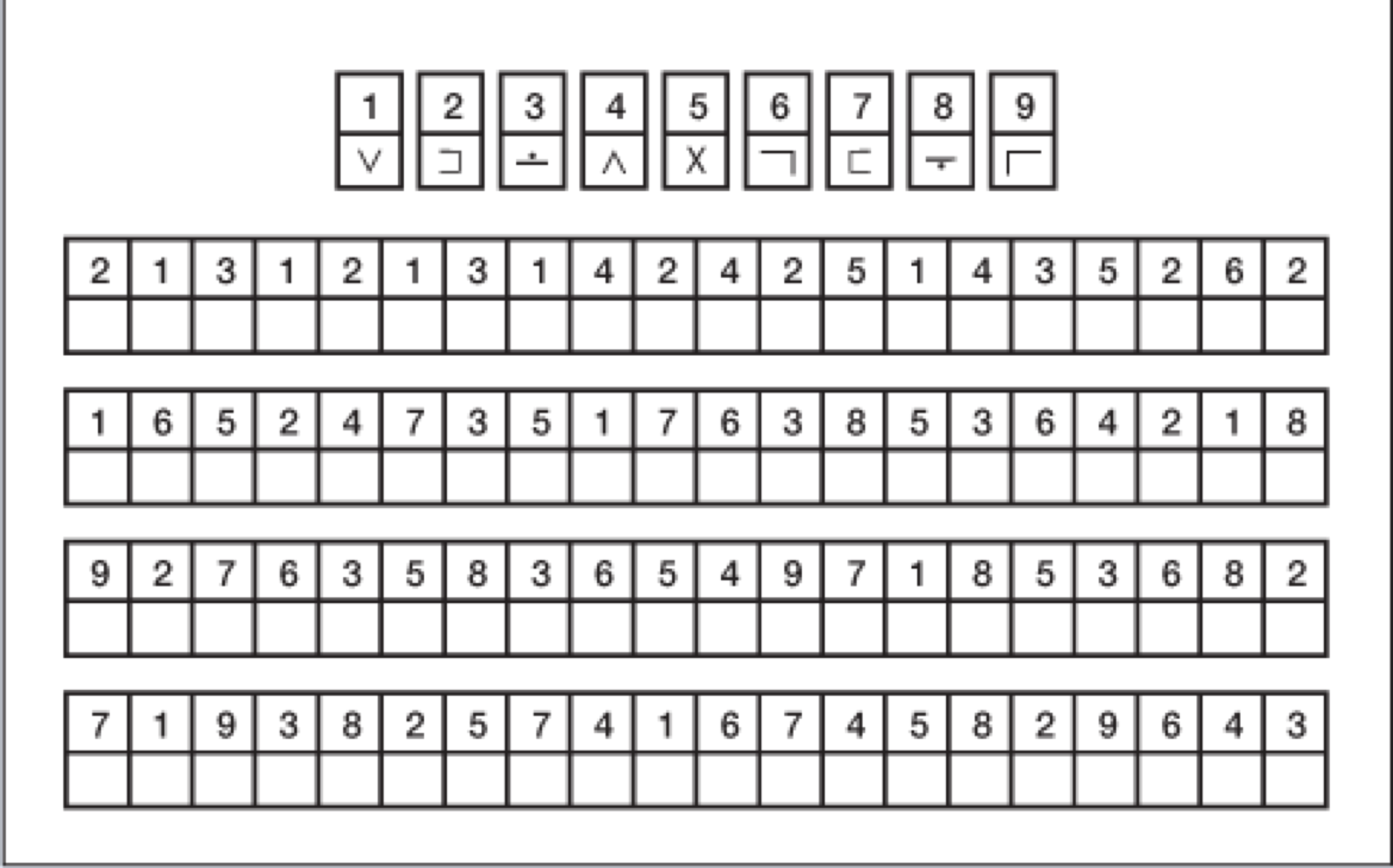
Figure 3. Digit Symbol Substitution Test (DSS).
Research Findings
Some of the first studies suggesting a link between hearing loss and cognition were conducted many years ago. They were simple, cross-sectional studies looking at a large group of people at one time point. The aim was to try and determine if greater hearing loss is associated with poorer cognitive scores, even after adjusting for factors like age, sex, race, education, etc.
In 2011, we conducted a study looking at 605 older adults in their 60s using the NHANES sample (Figure 4). We used the Digit Symbol Substitution Test. For every one-year increase in age, the DSS score is about .5 point lower. As the person's age goes up, their cognitive scores go down a bit. If you look at each 25 dB shift in the pure tone average, the score is about 3.9 points lower. The difference in age in years that is equivalent to a 25 dB hearing loss; seven years of aging is meaningful for a 25 dB hearing loss (Lin, 2011).
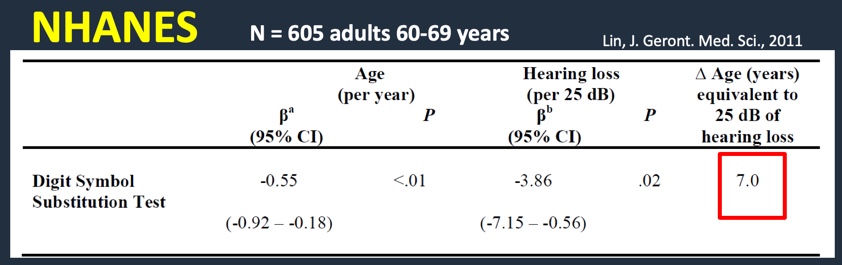
Figure 4. Research findings: NHANES sample using DSS (2011).
Since the hallmark of science is replication, a second study was done around the same time period looking at 347 adults using the BLSA dataset (Figure 5). These were adults without dementia over the age of 60. They all had their hearing measured. The tests conducted were the Stroop Mixed and the Trail Making B tests. We can see the same relationship between increasing years of age with poorer cognitive scores. The association was a 25 dB hearing loss. If you look at the last column, remarkably across two different studies and three completely different cognitive tests, you're seeing exactly the same results. A 25 dB hearing loss is roughly equivalent to almost seven years of aging (Lin et al., 2011). These findings suggest a definite link between hearing loss and reduced cognition.
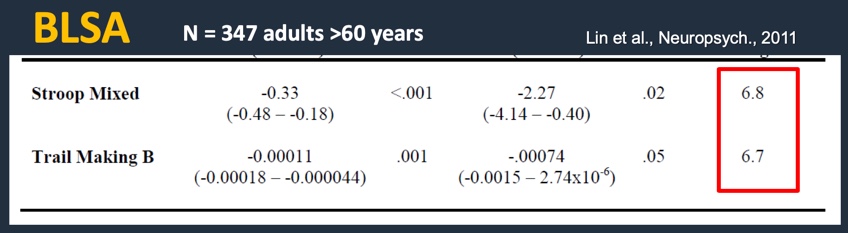
Figure 5. Research findings: BLSA dataset using Stroop Mixed and Trail Making B (2011).
Although this data seems promising, you may be thinking that these are just cross-sectional studies where everyone is measured at one time point. You may want to see some longitudinal data following the same group of people over many years to see whether those with hearing loss did, in fact, have faster rates of cognitive decline than their normal-hearing peers. In 2013, we conducted a follow-up study using data from the HealthABC study. We followed about 2,000 older adults over the age of 70 for six years. Subjects had their hearing measured in the beginning and had a modified mini-mental state score (3MS) and a Digit Symbol Substitution (DSS) score. We do those tests every year for many consecutive years in order to see how the trajectory is different between those with and without hearing loss. Again, we adjusted for age, sex, race, education, study site, smoking status, hypertension, diabetes, and stroke history (Figure 6).
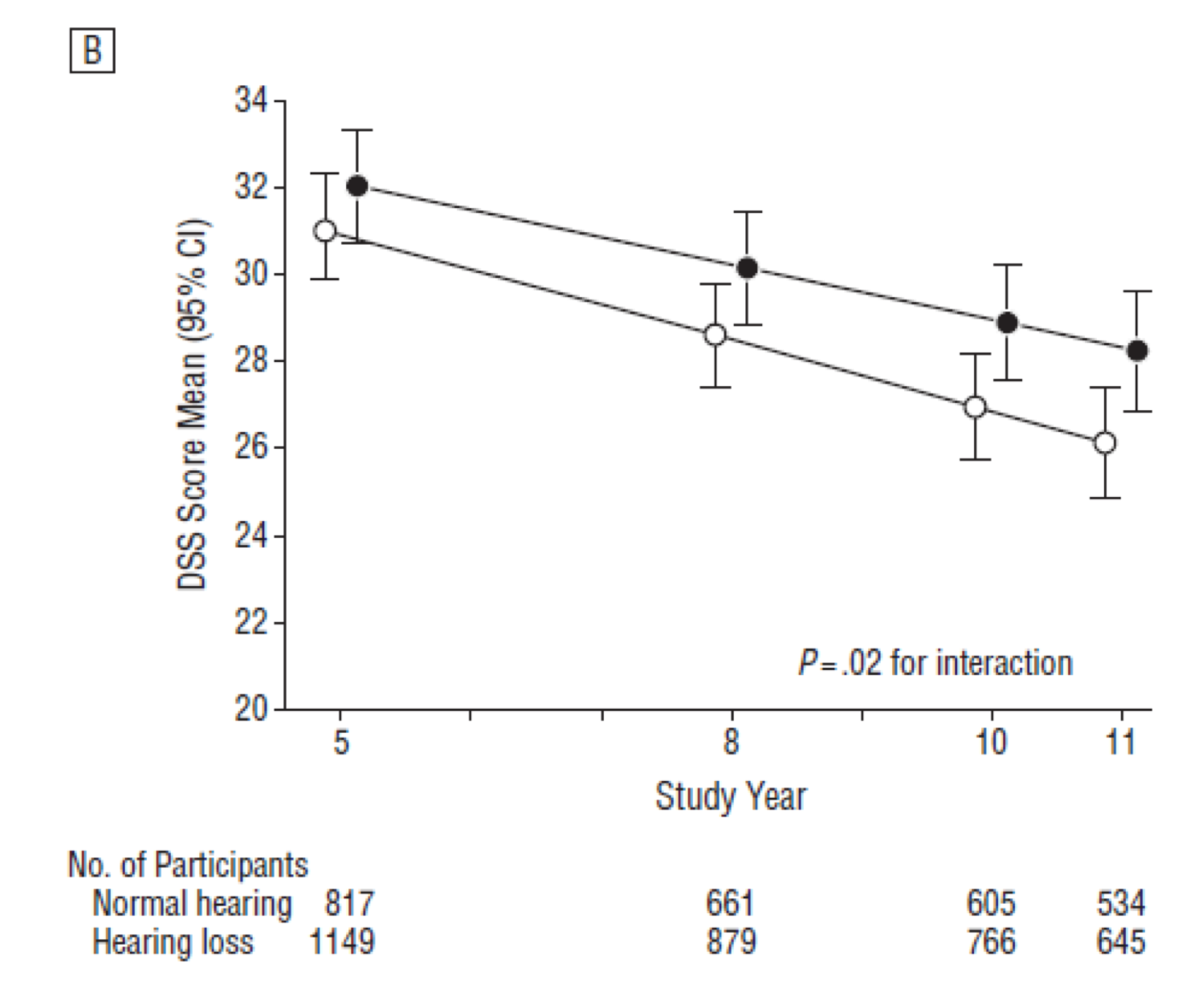
Figure 6. Research findings: HealthABC dataset using 3MS and DSS scores (2013).
These results are very much the same as the other studies we looked at. Year five is at the beginning of the study when the hearing testing was done. People with hearing loss have lower cognitive scores on the modified mini-mental state consistent with the previous cross-sectional data. Following them for the next six years, we see about a 40% faster rate of cognitive decline in the mini-mental in compared to those with normal hearing. If you apply the same analysis to the Digit Substitution Score, those with hearing loss have about a 30% faster rate of cognitive decline than those with normal hearing. Although not shown in Figure 6, these results indicate that there is a dose-dependent effect whereby the greater the hearing loss, the faster the rate of cognitive decline over time, even after adjusting for age, sex, race, education, study site, smoking status, hypertension, diabetes, and stroke history (Lin et al., 2013).
If you recall, I began the presentation today talking about aging and dementia. A person is considered to have dementia when their cognitive decline becomes so severe that they can no longer engage in daily activities. We do, in fact, see that people with hearing loss are at a greater risk of being diagnosed with dementia over time as compared to their normal-hearing peers. In a lot of these studies, dementia is a carefully adjudicated diagnosis, not based on one test or on one exam, but based on a series of things, such as laboratory studies, MRI studies, patient interviews, and psychometric interviews.
In this next study, we wanted to ascertain whether hearing loss is associated with a greater risk of being diagnosed with dementia over time (Figure 7). This is the raw data in the form of a summary plot, called a Kaplan Meier plot. The Y-axis is the percent of people who are dementia-free, and the X-axis acts as the time from baseline. At time zero, by definition, no one had dementia, which is why they were in the study. We followed them for the next 15 years. Looking at Figure 7, there is a clear breakdown showing that the severity of hearing loss at baseline was directly associated with the risk of being diagnosed and classified with dementia over time.
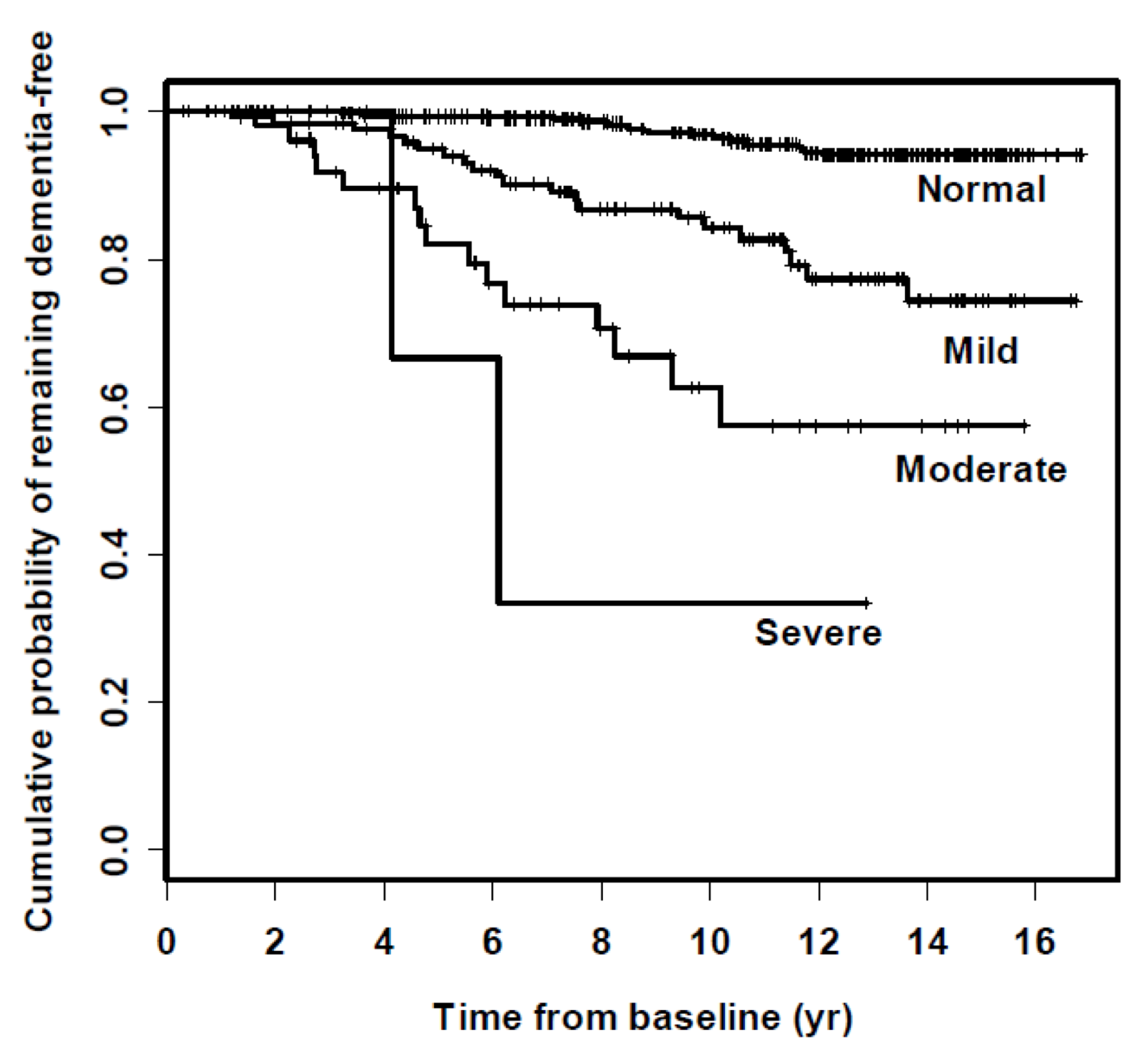
Figure 7. Hearing loss and incidence of dementia over time.
If we apply some statistical modeling to this data (controlling for age, sex, race, education, DM, smoking, and hypertension), we see that on average, compared to people with normal hearing, people with hearing loss are at increased risk of being diagnosed with dementia (Figure 8). The greater the degree of hearing loss, the greater the risk. Those with mild hearing loss were roughly twice as likely, those with moderate hearing loss were three times as likely, and those with severe hearing loss were almost five times as likely to be diagnosed with dementia as compared to normal hearing counterparts. These were substantial risk estimates, which, when we published this years ago, we honestly didn't believe it. The risk estimates were so strong that you typically don't see this in dementia literature (Lin et al., 2011).
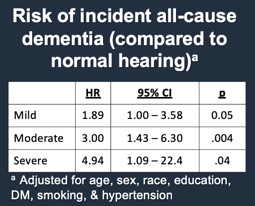
Figure 8. Risk of incident all-cause dementia (compared to normal hearing).
Again, the hallmark of good science is replication from other study sets. John Gallacher and his team in the United Kingdom followed over 1000 men for 17 years. In a fully adjusted model controlling for age, social class, etc., for every increase in 10 dB of hearing loss, the men had a 2.5 fold increased risk of being diagnosed with dementia over time. The fact that similar results were found across two independent studies with two completely different group investigators lends credence to the link between hearing loss and dementia (Gallacher et al., 2012)
Lancet Commission on Dementia Prevention, Intervention and Care
The best summary of this link between hearing loss and dementia was published in the Lancet, which as many of you know is a prestigious journal based in the United Kingdom. In 2017, they convened a formal commission of experts around the world to look at all the major risk factors for dementia. They named this group of experts the Lancet Commission on Dementia Prevention, Intervention and Care. Their goal was to help guide governments and societies around the world how to prioritize dementia research and determine what steps we should take to reduce the risk and possibly even prevent dementia.
The Lancet Commission conducted a large scale review of all the major extant literature. In the end, they concluded that about 65% of dementia risk involves non-modifiable factors that we can't do anything about or that we have no control over. They also estimated that about 35% of dementia risk is from potentially modifiable risk factors or risk factors that we can intervene upon.
The commission looked all the major risk factors for dementia from birth through late life. In addition to hearing loss, this list of risk factors includes the gene APOE e4 allele, as well as conditions such as low education, hypertension, obesity, smoking, depression, physical inactivity, social isolation, and diabetes. In the end, they identified hearing loss in mid and late life as the single most modifiable risk factor to prevent dementia, as compared to all other known risk factors. In other words, if hearing loss was prevented or treated, that could lead to a 9% reduction in the number of dementia cases down the road (Livingston et al., 2017). Needless to say, when this study came out, it made people sit up and take notice about the importance of treating hearing loss.
In summary, we have discussed some of the consequences of hearing loss from an epidemiologic standpoint. We have reviewed studies that have laid the foundation for our understanding of how hearing loss may, in fact, be related to this much more critical outcome of aging and public health. This lays out a whole new area of research that has not been previously explored in public health.
Impact of Treating Hearing Loss in Older Adults
The next question we need to ask is, "What is the impact of treating hearing loss in older adults?" Hearing loss may be associated with an increased risk of dementia, but if we treat the hearing loss, does that, in fact, make a difference in the incidence of dementia? Unfortunately, the state of the science is such that we have no idea. The question of whether treating hearing loss could delay cognitive decline or dementia remains unknown. Up until now, in the history of science, there has never been a randomized control trial conducted that has looked at the impact of hearing interventions on these broader, cognitive-related outcomes.
People will often ask me, in any of the previous epidemiologic studies that have been done, have any of the participants worn hearing aids, and if so, do those people do better than those without hearing aids? As one would imagine, on average, people with hearing loss who use hearing aids do better than those without. However, I cannot report this observation in the results because you can't believe it from a scientific standpoint. If you take two groups of people with hearing loss, and one group uses hearing aids and other group doesn't, those groups are fundamentally different. People who use hearing aids are likely to be more affluent, better educated, and more health conscience. All of those factors would lead toward them having better results anyway. If you observe people with hearing aids that are doing better than those without, is it because they got the hearing loss addressed? Or, was it because of the underlying factors that led them to get a hearing aid? That is something you can't tease apart from a simple observational study, and hence the importance of doing a clinical trial.
Based on the literature, if we now believe that there are mechanisms that link hearing loss with cognitive impairment and dementia, and we believe that existing interventions (e.g., hearing aids and cochlear implants) could directly help modify these pathways, we would hope that by providing a clear auditory signal we are reducing cognitive load, providing increased auditory brain stimulation, and improving social function in mid to late life. This is the reason why hearing loss has gotten so interesting from the standpoint of public health and has received a lot of public and media attention. It is because of the possibility of hearing loss being a modifiable risk factor in late-life for cognitive decline and dementia. This is important because other risk factors (e.g., high blood pressure and diabetes) typically need to be addressed and require intervention much earlier in life. If a 75-year-old woman with untreated high blood pressure and diabetes comes to your clinic today worried about dementia, it may be too late for treatment to have an impact on the onset of dementia. However, if she, like two-thirds of the older adult population, has hearing loss, providing intervention late in life may still offer a benefit as it relates to the prevention of cognitive decline and dementia.
Clinical Trial: Aging and Cognitive Health Evaluation in Elders (ACHIEVE)
In response to the potential cognitive benefit of late-life treatment hearing loss, there is a clinical trial that is being currently conducted in the United States at four different study sites. This nearly $20 million dollar study is funded by the National Institute of Health. It's called the ACHIEVE study, or the Aging and Cognitive Health Evaluation in Elders. In this study, 850 older adults with some degree of hearing loss will be randomized in the very beginning. They'll be randomized based on hearing intervention, or they'll receive one-on-one sessions with a health educator to cover topics around healthy aging and nutrition, exercise, etc. They're getting exposure to study personnel, but it's definitely getting their hearing addressed or addressing other aspects of healthy aging education. They will be followed for the next three years. The primary outcome of the study is to see if, after three years of hearing loss intervention, rates of cognitive decline are slower and the risk for dementia has been lowered. Additionally, there are a whole host of secondary outcomes to look at in this study, including dementia, social and leisure activities, physical functioning, mobility (measured with accelerometry and objective, self-report measures), falls and hospitalizations, and also structural brain MRI measures.
The main inclusion criteria for the ACHIEVE trial participants are:
- 70-84 y.o. community-dwelling adults
- Mild-moderate hearing loss
- No cognitive impairment
- No self-reported disability in >1 ADL
- No self-reported hearing aid use in the past year
There are two different interventions being used in the study. The hearing intervention has been operationalized by colleagues at the University of South Florida. It is a best practice hearing intervention consisting of four sessions with a study audiologist where they're receiving hearing loss education, as well as hearing aids, remote devices, and whatever they need to help them communicate better. In contrast, the other group will be getting an established program for successful aging developed over many years with the University of Pittsburgh. This second group will receive four sessions with a help educator learning about the 10 Keys program. Developed by the Centers for Disease Control, the 10 Keys program is a proven education program to promote healthy aging, and covers issues like nutrition, exercise, cancer screening, etc.
In the study, everyone gets a baseline and semi-annual visits for intervention delivery and outcome assessments every six months. The primary outcome is global and domain-specific cognitive function. Participants will undergo a one-hour neurocognitive battery of tests to measure the different domains of cognition. As stated earlier, there are many additional secondary outcomes. Ultimately, the study aims to determine whether rates of cognitive change/decline can be slowed down over a three-year period, comparing those who are treated vs. untreated.
The timeline for the ACHIEVE Trial is as follows:
- 2011- 2013
- Epidemiological studies establishing the association between hearing loss & dementia
- 2014 - 2016
- Trial planning grant (NIH R34AG046548)
- Pilot study, development of protocol/operations manual, submission of grant for full-scale trial
- 2017
- ACHIEVE grant funded by the National Institute on Aging (R01AG055426)
- 2018 - 19 Recruitment at field sites
- 2019 - 22 Follow-up
A trial like this can be a little daunting in terms of the time scale and scope in that we will not have final, definitive results until the tail-end of 2022. A clinical trial like this takes time because you're looking at rates of change over time, which requires you to follow people for many, many years.
Effectively Addressing Hearing Loss in Society
The third question I want to touch on today is since we don't yet have the evidence of the clinical trial, what do we do in the meantime? How can hearing loss be effectively addressed in society? For the purposes of today's presentation, I'm going to focus on things that we can do individually as hearing healthcare providers.
First, what is the goal when seeing a patient with hearing loss? You may offer answers like "It's to make sure the patient understands how a hearing aid will help them" or "To ensure that the hearing aid is properly programmed with Verifit measures." While these are reasonable answers, I always like to take a step back and realize that there is a much more fundamental premise centering around making sure the patient can communicate effectively in all settings. That's the ultimate goal. Someone has hearing loss, you want to make sure they can communicate effectively. That's all it is.
Ensuring Effective Communication
In order to ensure effective communication, there are a couple of major approaches. The first and arguably most important approach is to review effective communication strategies. This may even be more important than any type of technology we can offer patients. In conversations with family and loved ones, as well as out in the community, our patients need to be able to follow some basic communication strategies.
Communication Strategy #1: Get face to face. Anytime our patients are face to face with someone they want to talk to and they are in a quiet room, they're likely going to hear fine. If you're speaking clearly in a quiet room, and the auditory signal comes in clearly enough to the ear, by the time the signal reaches the brain, it should be good enough to allow the person to hear it. In addition, visual cues such as facial expressions and gestures help fill in any gaps. Also, turning off background any noise helps with communication.
Communication Strategy #2: Repeat then reword. This is a strategy that is useful for the communication partner of a person who is hearing impaired. If someone did not understand you, repeat the question or statement once. If the person with hearing loss still cannot understand it, rather than yelling it a third time, reword the statement or direction. The burden for this strategy is not on your patient, but with their communication partner. As such, it is helpful if you are able to counsel not only the patient but perhaps their spouse or family members.
Communication Strategy #3: Summarize. The third strategy is for the patient to be specific about what they do and do not hear. Rather than saying "huh" or "what", if is more helpful if they summarize and rephrase what they heard or what they missed in the conversation.
Choosing the Right Technologies
Now that we've given our patients some effective communication strategies, we need to help determine the best technologies for them.
Hearing aids. Clearly, hearing aids are the stepping stone for a lot of people. In some cases of more moderate to severe hearing loss, I always emphasize that it's not just a hearing aid, but it's also about the importance of being able to use accessories, such as remote mics and streamers, to get the signally clearly.
Cochlear implants. As an implant surgeon, the rule of thumb I follow is anytime I see a speech discrimination score of less than 60% in quiet, those people deserve CI referral. Not all of them will be eligible because the CI testing criteria are a little different. Too often, I see that's not the case, but we owe it to our patients to make sure they at least get evaluated for a CI.
Assistive listening devices. Obviously, telephone use is a big issue with most of our patients. Skype and FaceTime can be useful because a classic landline phone signal is clipped between 500 and 4000 before assisted bandwidth issues. Tools with a wider bandwidth that use the internet such as Skype and FaceTime result in a clearer auditory signal. Another choice for patients who use the phone is to use captioned phones. Captioned phones allow our patients to better hear what's going on because they have a mix of voice recognition as well as a caption agent which will transcribe the caller's voice. Many times, the cost of captioning services is completely covered. Captioned phones are funded by a federal program which was established under the Americans with Disabilities Act. Oversight is provided by the Federal Communications Commission (FCC). In many cases, qualifying patients can receive a captioned phone system and service for free or at low cost. Figure 9 shows CaptionCall's classic example of a captioned phone.

Figure 9. CaptionCall captioned phone.
Portable amplifiers. Beyond assistive listening devices, portable amplifiers are useful for patients who already have dementia or patients for whom a hearing aid is not the right choice (Figure 10). For some people, a hearing aid is too small and they get lost. Sometimes, for patients with mild cognitive impairment, they want something simple to use, like a basic, body-worn amplifier. These can be great options for our patients.

Figure 10. Examples of portable amplifiers.
OTC hearing aids. Lastly, we have all likely heard about over-the-counter hearing aids. Current FDA and state regulations limit hearing aid sales only through a licensed professional, such as an ENT, audiologist or hearing aid dispenser. However, over the last several years, based on recommendations from the White House President's Council of Advisors on Science and Technology and the National Academies, the OTC Hearing Aid bill was passed into law in August 2017. By August 2020, the FDA will issue final regulations and performance standards to completely re-regulate hearing aids to ensure safety and effectiveness. Regulations will allow any manufacturer meeting the regulations and standards to directly sell hearing aids over the counter to consumers. As a result of these new regulations, we will be seeing a convergence of medical devices (e.g., hearing aids) with consumer electronics (i.e., "hearables"). Once the final regulations are issued, the entire dynamic of what hearing technology means will be changed, including who can access it, how people access it, even the pricing.
Summary and Conclusion
In summary, as we begin thinking about how to address hearing loss, the fundamental, most important technique we can share with our patients is to use effective communication strategies. In addition, there are many different types of technologies that we can offer to help patients hear better. As our ultimate goal is to help our patients communicate better and more effectively, we may recommend a combination of techniques and technologies. For one patient, a well-fit traditional hearing aid will do the trick. Other patients may be candidates for a cochlear implant. Still others might benefit from something as simple as a PockeTalker, a portable amplifier, a captioned phone, or -- coming soon -- an OTC hearing aid.
Medical science has advanced tremendously in the last few decades. Even in the early 1990s, hypertension in older adults was routinely ignored and seen as a normal process of aging. We now know that hypertension needs to be treated to reduce the risk of heart attack and stroke. Similarly, although hearing loss is a normal process of aging, we now understand that untreated hearing loss could increase a person's risk for cognitive decline or dementia. By the end of 2022, we are optimistic that the ACHIEVE clinical trial will ultimately serve to shed more light on the link between hearing loss intervention and a reduction in cognitive decline.
If you are interested in learning more about what we do, the website address for the Johns Hopkins Bloomberg School of Public Health and the Cochlear Center for Hearing and Public Health is www.jhsph.edu/cochlear-center.
References
Cole, S. W., Hawkley, L. C., Arevalo, J. M., Sung, C. Y., Rose, R. M., & Cacioppo, J. T. (2007). Social regulation of gene expression in human leukocytes. Genome biology, 8(9), R189.
Cole, S. W., Hawkley, L. C., Arevalo, J. M., & Cacioppo, J. T. (2011). Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proceedings of the National Academy of Sciences, 108(7), 3080-3085.
Fries, J. F. (1982). The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol, (9)789-793.
Gallacher, J., Ilubaera, V., Ben-Shlomo, Y., Bayer, A., Fish, M., Babisch, W., & Elwood, P. (2012). Auditory threshold, phonologic demand, and incident dementia. Neurology, 79(15), 1583-1590.
Hebert, L. E., Weuve, J., Scherr, P. A., & Evans, D. A. (2013). Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology, 80(19), 1778-1783.
Kahneman, D. (1973). Attention and effort (Vol. 1063). Englewood Cliffs, NJ: Prentice-Hall.
Lin, F. R., Metter, E. J., O’brien, R. J., Resnick, S. M., Zonderman, A. B., & Ferrucci, L. (2011). Hearing loss and incident dementia. Archives of neurology, 68(2), 214-220.
Lin, F. R., Ferrucci, L., Metter, E. J., An, Y., Zonderman, A. B., & Resnick, S. M. (2011). Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology, 25(6), 763.
Lin, F. R. (2011). Hearing loss and cognition among older adults in the United States. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 66(10), 1131-1136.
Lin, F. R., Niparko, J. K., & Ferrucci, L. (2011). Hearing loss prevalence in the United States. Archives of internal medicine, 171(20), 1851-1853.
Lin, F. R., Yaffe, K., Xia, J., Xue, Q. L., Harris, T. B., Purchase-Helzner, E., ... & Health ABC Study Group, F. (2013). Hearing loss and cognitive decline in older adults. JAMA internal medicine, 173(4), 293-299.
Lin FR, Albert M. (2014). Hearing loss and dementia - who is listening? Aging Mental Health 2014, 18(6):671-3.
Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D., ... & Cooper, C. (2017). Dementia prevention, intervention, and care. The Lancet, 390(10113), 2673-2734.
Nations, U. (2015). World population prospects: The 2015 revision. United Nations Econ Soc Aff, 33(2), 1-66.
Peelle, J. E., Troiani, V., Grossman, M., & Wingfield, A. (2011). Hearing loss in older adults affects neural systems supporting speech comprehension. Journal of Neuroscience, 31(35), 12638-12643.
Citation
Lin, F. (2019). Hearing loss & aging: a public health perspective. AudiologyOnline, Article 24436. Retrieved from https://www.audiologyonline.com


