Editor's note: This is an edited transcript of a live webinar. Download supplemental course material here.
Gus Mueller: I am excited to be here today and am looking forward to this presentation. The Vanderbilt Audiology Journal Club is presented four times a year on AudiologyOnline, and this is our fourth year. Each journal club features a member of the Vanderbilt audiology faculty discussing key journal articles around a particular topic aligned with his or her clinical or research interests.
Let me introduce our presenter today. Dr. René Gifford is one busy person. Not only does she work with Vanderbilt audiology students, but she conducts a vast array of research as well, as Director of the Cochlear Implant Research Laboratory at Vanderbilt. It seems like every time I turn around at a professional meeting, I see a poster or a presentation that includes René or one of her students. René is an assistant professor of audiology at Vanderbilt. She is also the Director of the Cochlear Implant Program and the Associate Director of Pediatric Audiology at the Vanderbilt Bill Wilkerson Center. René, I am going to turn it over to you.
René Gifford: Thank you, Gus. Today we are going to talk about hearing preservation with cochlear implants. Figure 1 shows a hypothetical audiogram of a patient we may have seen for a cochlear implant in the earliest days of cochlear implants. This was back when the Food and Drug Administration (FDA) indications for a cochlear implant required individuals to have bilateral, profound sensorineural hearing loss, no open-set word recognition, and post-lingual onset of deafness. Most of those patients had no behavioral responses to sound whatsoever. My, how things have changed since then.
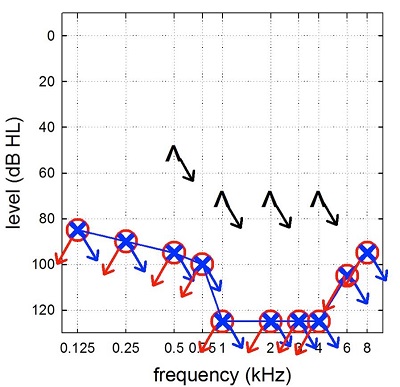
Figure 1. Example of an audiogram from an early-generation cochlear implant candidate. Note that there is bilateral, profound sensorineural hearing loss.
Figure 2 is typical of the audiograms of the cochlear implant candidates I now see weekly in the audiology clinic and my research lab. We see patients who have moderate, sloping to profound sensorineural hearing losses. Some patients with these type of hearing losses do quite well with hearing aids and others have very poor word recognition with hearing aids regardless of the amount of amplification you provide for them.
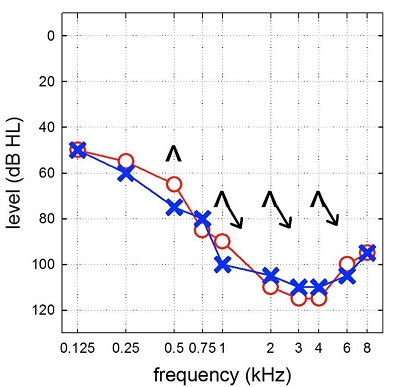
Figure 2. Example of an audiogram from a modern cochlear implant candidate, consistent with moderate, sloping to severe-to-profound sensorinueral hearing loss.
Figure 3 is an audiogram of a patient that is enrolled in one of my research studies. You can see that hearing is essentially normal or near normal at the lowest frequency, then slopes precipitously to a profound sensorineural hearing loss.
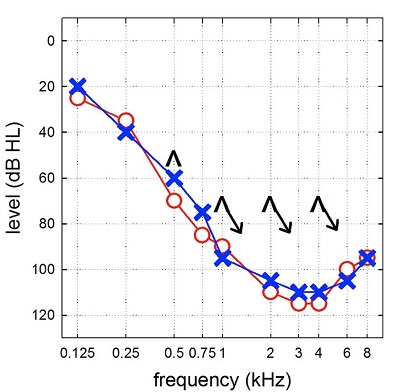
Figure 3. Audiogram demonstrating normal hearing sloping to profound sensorineural hearing loss bilaterally.
When you see these audiograms, there are a number of potential overlapping indications. We have to determine the very best treatment option for the patient sitting across from us. Is it hearing aids? Is it cochlear implants? Is it a cochlear implant and hearing aids? Is it a middle ear implant? Perhaps it is a combination thereof, such as electric and acoustic stimulation (EAS), which would be a hybrid cochlear implant with acoustic stimulation.
There are some underlying issues regarding fitting hearing aids to these patients. We know that people who have precipitously sloping hearing losses are very difficult to fit with hearing aids. There are many potential reasons for this. Fittings have become better in recent years with the advent of receiver in the canal (RIC) hearing aids. However, there are a number of studies that have shown a high likelihood of cochlear dead regions in hearing losses like this. Vinay and Moore (2007) looked at almost 600 ears. They found cochlear dead regions in nearly 60% of subjects when thresholds were in excess of 70 dB HL. That means that you could give these patients the very best, well-fitted, premium hearing aids, but there are no primary auditory neurons to transmit that information to the central auditory system so there is limited benefit. That is where we start thinking about cochlear implants.
Figure 4 shows a very crude schematic of the functioning of a conventional cochlear implant system. The top bar represents the basilar membrane, uncoiled from the apex to the base. Low frequencies are at the apex and increase in frequency toward the base. The bar directly below shows a series of overlapping band-pass filters representing the peripheral filtering that takes place along the basilar membrane. We assume that there are few surviving inner or outer hair cells, so a cochlear implant electrode array is inserted, and the individual then hears through electric pulses.
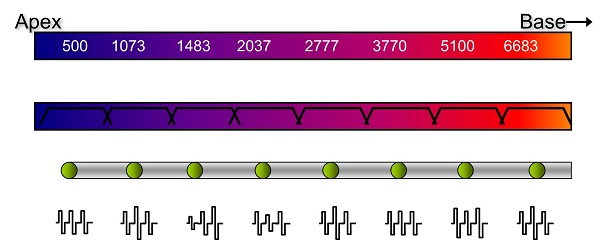
Figure 4. Schematic of a conventional cochlear implant array, from apex to base.
Cochlear Implants: Hearing Preservation
Today we are talking about combined electric and acoustic hearing, for individuals with considerable residual low-frequency hearing. With normal or near-normal low-frequency hearing, the presence of good inner and outer hair cells is very likely. We want to preserve that hearing as best we can. In these cases, the surgeon would partially implant an electrode array into the cochlea with the hopes of preserving the cochlear structures apical to the tip of the array; the mid to high frequencies would be transmitted electrically, meaning that this individual would hear both acoustic and electric stimuli in the same ear.
In the past, we had to counsel people getting cochlear implants that they had to be prepared to sacrifice any residual hearing as a result of the cochlear implant surgery. That is not necessarily the case anymore. In fact, we are seeing considerable preservation of acoustic hearing with both short and long electrodes. How can this be? It is primarily due to two factors, minimally traumatic surgery and atraumatic electrodes.
Minimally Traumatic Surgery
Minimally traumatic surgery used to be called “soft surgery.” Surgeons did not like that term, because there is nothing soft about surgery. Minimally traumatic surgery means that the cochleostomy, which is a hole drilled into cochlea through which the electrode is inserted, remains as small as possible, and the size and the location is in the right place.
A cochleostomy should go directly in the scala tympani. You can completely forego a cochleostomy and insert the electrode through the round window, which opens directly into the scala tympani. In fact, there is research showing that round window insertion, particularly for the straight electrodes, is the way to go. Whether you go through a cochleostomy or the round window, you have to open the endosteum to access the cochlea. Years ago, they used a diamond burr and drilled right through. When you started to see the perilymph emerging from the cochlea you knew you had broken through the endosteum. That is no longer the case. Now they take very good care about opening the endosteum as minimally as possible, ensuring that no blood or bone dust gets in.
Hyaluronic acid or Healon® is used in cochlear implant surgeries today. It works as a lubricant. It also works to seal the opening to the cochlea if you are not immediately ready to insert the electrode. When the surgeon places the Healon on the opening, the fluids are kept intracochlearly. When the electrode is inserted, it can pass smoothly through the opening. You want to be careful about allowing too much perilymph to escape out of the cochlea, because we would not want to change the endocochlear potential. Surgeons also have to be aware of the electrode insertion force and speed, keeping it slow and steady. We know that force equals trauma.
Steroids are a factor in minimally traumatic surgery as well. One of the papers that we will talk about later in this presentation focuses on steroids. Steroids are a hot topic in the literature and among surgeons regarding hearing preservation. There is ongoing discussion about how much steroid should be administered preoperatively, postoperatively and perioperatively.
Atraumatic Electrodes
For the first time in a number of years, we have a variety of electrodes at our disposal; surgeons and audiologists can work together to select the perfect individualized electrode for a patient. Looking at the cochlear duct length on the preoperative CT scan can help determine length, as can identifying how much hearing there is to preserve. Long, medium and short electrodes are all options at our disposal.
One example of an atraumatic electrode is the Cochlear Nucleus CI422. The tip of the electrode is 0.3 mm in diameter. Realistically, that fits easily through a round window or small cochleostomy. This is a very thin electrode. Even at its most basal edge, it is only 0.6 mm. This would be considered a small, flexible, atraumatic electrode.
MED-EL makes both standard and atraumatic electrodes. The standard electrode is what we would consider for the conventional cochlear implant recipient. It is the longest electrode available for complete cochlear coverage. It is designed to be inserted up to 31 mm and covers almost the entire cochlear duct.
The MED-EL Flex28 is shorter, and thinner at its apical edge. This electrode has some thinning towards the apex, allowing for an atraumatic insertion. The MED-EL Flex24 is available for someone who might have even more hearing to preserve; it is very similar to the Flex28, but is slightly shorter. The insertion depth is also a bit more shallow.
Hybrid electrodes are not available to the commercial public, but are being used in clinical trials. Close to 90 patients are implanted in the U.S with the Nucleus Hybrid S8, which is no longer available. This was a 10 mm insertion with six intracochlear electrodes. There now is the Hybrid S12, which also has a 10 mm insertion, but instead of six electrodes spaced over the distal edge, there are 10 electrodes spaced over the distal edge. It provides more stimulating electrodes in the cochlea.
Lastly, Cochlear offers the Hybrid L24, which is designed for up to an 18 mm insertion. There are 22 electrodes spaced along the distal edge. You have the exact number of electrodes in the L24 as you would in a standard Nucleus cochlear implant system. As I mentioned, you select the electrode based on the individual patients. For example, if you have someone with residual low-frequency hearing and you are concerned about the effects of too deep of an electrode insertion, you might select a Hybrid S12. If you have someone for whom you want to provide a little more electrical stimulation along the cochlea, you might select the L24. It is very exciting to be working clinically and in research at this time due to all the options that are available.
The information behind cochlear implants and hearing preservation is exciting, but there are a number of skeptics, including surgeons, audiologists and patients. I have heard statements such as, “Hearing preservation does not matter, because the residual hearing is useless. The patient is not able to take advantage of the acoustic hearing to achieve high levels of speech recognition.” The next thing I hear most commonly is, “My patients do well with their cochlear implants, and we do not preserve hearing. My patients perform on average or above average. Why would I take more time to try to preserve hearing?” Minimally traumatic surgery does take more time, and in the operating room, time equals money. The surgery itself also is a little more difficult, so it takes time and more effort.
Skeptics also bring up the issue of losing hearing over time. There are a number of studies that have shown that patients lose about 1 dB per year over the next 10, 20, or 30 years. Do we go to all this effort to preserve hearing that may not be left in 20 years? I also hear, “We are setting ourselves up for failure, because we cannot provide a guarantee that we will preserve hearing. Certainly we will do our best to preserve residual hearing and provide them electric and acoustic hearing postoperatively, but in the end, we might fail.”
Research: Hearing Preservation
Let’s look at the research. The main question of interest to me is, “Does hearing preservation improve speech recognition outcomes?” If we can determine efficacy for hearing preservation, then I would say to the skeptics that it is worth the effort to at least attempt hearing preservation.
The first paper I would like to discuss is by Rader, Fastl and Baumann (2013). They examined 44 subjects, 22 of which were normal-hearing controls. They had 10 individuals who were bilaterally implanted and 12 individuals who had hearing preservation with the cochlear implant. Of those 12, 11 were implanted with the Flex EAS electrode, which is now marketed as the MED-EL Flex24. The other 12 patients were implanted with the Flex20, which is not currently available in the United States, but has shallower insertion than even the Flex24. All of these electrodes are straight, atraumatic electrodes.
Methods
The individuals in the study who were implanted had considerable hearing preservation mean thresholds in the implanted ear, consistent with a mild-sloping-to-profound sensorineural hearing loss. Their postoperative thresholds looked similar to the thresholds in the unimplanted ear. They had aidable hearing at least through 250 Hz, and many would argue through 500 Hz as well. There was much hearing that was aidable, even in the implanted ear postoperatively.
The researchers used the Fryberger monosyllables, which are typically used in Germany where this study was conducted. They compared EAS patients in a bimodal arrangement (cochlear implant plus hearing aid on the contralateral ear) to a hearing alone on the unimplanted ear to bilateral cochlear implant users. Even with relatively good hearing in the nonimplanted ear, patients performed rather poorly with the contralateral hearing aid alone. These are patients who are not doing well with their acoustic hearing, even with appropriately-fitted hearing aids. Statistically speaking, the EAS in the bilaterally implanted patients did not perform differently. This means that it did not matter if you had a single implant and bilateral acoustic hearing or two cochlear implants. All of those patients achieved about the same word recognition performance. If you only had these data and nothing else, someone could easily say that the subjects with bilateral cochlear implants did not have any hearing preservation and did just as well as the subjects with bilateral acoustic hearing preservation. However, I would say this is a very crude estimate of speech recognition because we are only assessing monosyllabic word recognition in quiet with a single loudspeaker placed directly in front of that patient. This is a best-case scenario, and it is unrealistic of what we encounter in the real world.
In the real world, we tend to have diffuse noise coming at us from a number of different angles. Our auditory system is continuously analyzing interaural time differences (ITD) cues in the presence of that kind of noise. When we have preserved low-frequency acoustic hearing, the combination of the ITDs for the noise and the lack of ITDs for the speech could be very beneficial, at least in theory. As adults, we tend to look at the individual to whom we are speaking, so speech should be reaching the two ears at the same time. Our auditory system is able to take advantage of ITDs to suppress the noise and extract the speech signal. That was the hypothesis for this study.
Rader, Fastl and Baumann (2013) used two different noise conditions. The first was speech at 0 degrees azimuth and noise at 0 degrees azimuth. This is the typical arrangement that we see in most of our clinics for speech recognition testing. Then they also used what they called the multisource noise field, or MSNF. For this condition, they used four loudspeakers placed in the corners of the room, as shown in the schematic in Figure 5. They presented noise from a fifth speaker, not shown in the schematic, at 0 degrees azimuth. They had two different noise conditions. Remember, they did use normal-hearing controls. They elevated the level of the noise to 75 dB SPL for normal-hearing listeners, but they were concerned about the level being too high for the implanted listeners, so they kept that fixed at 65 dB SPL for the listeners with cochlear implants. This was a fixed-noise-level condition, in which the speech was varied adaptively. That gives us a speech reception threshold or the signal-to-noise ratio (SNR) required to get about 50% correct. In this case, lower numbers are better scores.
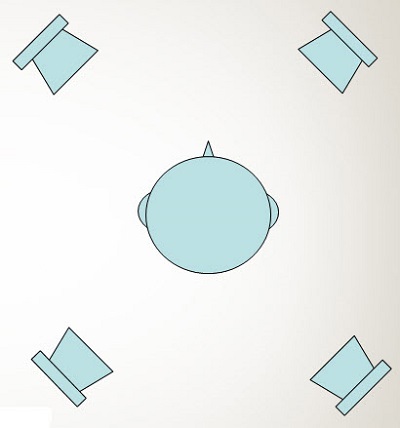
Figure 5. Four-speaker arrangement for speech perception testing.
The researchers used three different kinds of noise. First was OLSA noise, which is what is given in the OLSA sentences, which are very similar to the Hearing in Noise Test (HINT) sentences. Then they have the CCITT noise, which is the Consultative Committee for International Telephony and Telegraphy. This is a steady-state noise that is speech weighted. It is very similar to what we would use with the HINT sentences. Lastly, they used Fastl noise, which is what I would call speech-correlated noise. They had the speech-weighted steady-state noise, but then they modulate that noise with the envelope of a single talker. In essence, there is noise that is fluctuating with the envelope of a single talker, but it also happens to be speech shaped. They use that noise so that the listeners did not suffer from the effects of informational masking, which you would have if you used a single talker.
Results
When the speech and noise are at 0 degrees azimuth in this S0N0 condition, the bilateral and EAS patients tended to perform more similarly in all cases. For the OLSA noise condition, the two groups performed almost the same. When using the CCITT, the groups showed more separation in scores. The same thing happened with the Fastl noise. These two groups performed more similarly, although the trend was that the EAS patients did perform better overall. As I said previously, this is a relatively unrealistic condition because it is very rare that noise originates from a signal source.
They next examined what happened when the noise was more diffuse in nature, as presented from the multisource noise field. For the OLSA noise, which is a steady-state speech-shaped noise, although the EAS patients did perform better that the bilaterally-implanted group, the lower interquartile range overlapped with the upper, which indicates no significant difference between the groups. The CCITT noise, which is a bit more complex than the OLSA noise, was also evaluated. The results showed that the subjects’ scores started separating out a bit more, with the EAS patients performing at a higher level than the bilaterally-implanted subjects.
When we look at the results using the Fastl noise, we start to see more overlap between scores from the EAS patients and the normal hearing controls. With this more realistic noise, individuals are able to take advantage of the interaural time difference cues and the temporally-fluctuating masker, allowing for listening in the dips. The individuals with low-frequency acoustic hearing in both ears appear more normal, and they are separating themselves from those individuals who do not have preserved hearing.
Limitations
One limitation of the study is that they only tested the best condition for the EAS subjects. In reality, we do not know if they would have performed just as well if we had plugged the acoustic-hearing in the implanted ear. Because the subjects had fairly good hearing in the non-implanted ear, we do not know if we can attribute the outcome effects to having preserved hearing in the implanted ear. Second, there was a small sample size - only 12 EAS patients and 10 bilaterally-implanted patients. We are unaware of how a larger, more heterogeneous sample might have affected the results.
Research: Speech Recognition
The next research question I have is, “Does hearing preservation improve speech recognition, and if it does, what is the underlying mechanism responsible for that benefit?” I am going to present on paper on which I was one of the authors, simply because we were able to include a larger sample size.
Subjects
This was a multi-center study that we worked on for several years (Gifford et al., 2007). We started collecting data in 2007, and it was published just last month. We included 54 subjects and 16 normal-hearing controls. Seventeen of these patients came from Professor Skarzynski’s group in Warsaw, and the remaining 21 were English speaking individuals. The Polish speaking individuals were all implanted with the MED-EL Flex EAS electrode, and the English speaking subjects had varied implant electrodes. We had two patients who were implanted with a standard MED-EL Sonata H electrode, which is up to a 31 mm insertion. We had two subjects with the MED-EL Flex EAS and 10 with the Nucleus Hybrid implant; six had the S8 and four had the long L24 array. We had 7 patients who were implanted with conventional perimodiolar electrodes, such as the Nucleus CI24RCA, the Freedom Contour Advance, and the 512 series.
We observed considerable hearing preservation both with short and long electrodes, as well as with straight and perimodiolar electrodes. It is possible. If you compare the low-frequency pure-tone average (125, 250, and 500 Hz) pre and postoperatively, the Polish and English groups had approximately a 20 dB loss of hearing in the low frequencies. This is consistent across the two language groups. This was interesting, because the majority of the English-speaking individuals had cochleostomies, whereas all of the Polish-speaking individuals were implanted via a round window approach. So we are seeing hearing preservation at similar rates with both surgical options as well.
Methods
In this study (Gifford et al., 2007), we used CNC monosyllabic words scored in percent correct for seven different listening conditions: ipsilateral hearing aid, contralateral hearing aid, bilateral hearing aids, cochlear implant alone, cochlear implant plus ipsilateral hearing aid, cochlear implant plus contralateral hearing aid, and best EAS. These are just for the English speaking subjects. There are 22 subjects included, because we have enrolled another individual since this paper was published. In the ipsilateral hearing aid in the implanted ear, contralateral hearing aid, and bilateral hearing aid conditions, they are still doing quite poor. When we test speech recognition with the implant by itself, we see a big jump in performance. We see another jump in performance when we add some acoustic hearing. We see about the same level of performance with the addition of the ipsilateral hearing aid, the contralateral hearing aid, or both. A skeptic might say, “We have people with bilateral acoustic hearing, and it does not matter what acoustic hearing they have; they are going to do the same. Why do we need to worry about preserving hearing?” Let’s look further into the research.
In our experiments, we had a restaurant simulation using the Revitronix R-SPACE system, which uses an eight-loudspeaker array. We also used an adaptive speech reception threshold test with an adaptive SNR. The noise was fixed at 72 dBA for the realistic restaurant noise condition. We also had a fixed level SNR, because in the real world, the speaker would not raise and lower the level of their voice based on whether or not you got the last sentence correct. We used a fixed SNR of +6 dB and +2 dB; the latter is what you would realistically expect with this level of background noise. We used sentences for this experiment. For the Polish-speaking group, we used the Polish Matrix Sentence Test (PMST), and for the English-speaking group, we used the HINT. We included reverberant speech recognition as well, along with a measurement of ITD thresholds. I will address those in a moment.
The best aided EAS condition was the implant plus bilateral hearing aids. We also tested the bimodal condition, in which we occluded the implanted ear so that we could tell whether or not adding this ear in the other condition made a difference for this patient. It is important to recognize that we did verify all hearing aid settings prior to testing, and we ensured that participants had speech recognition at 60 dB SPL, which is minimally required for our clinical protocols.
Figure 6 is a photo of one of my past research assistants and now audiologist, Amy, who is sitting in the R-SPACE system. You can see the eight loudspeakers placed all around the subject. In our study case, the speech originated from the front speaker and the noise came from all eight speakers, including the speaker from the front.
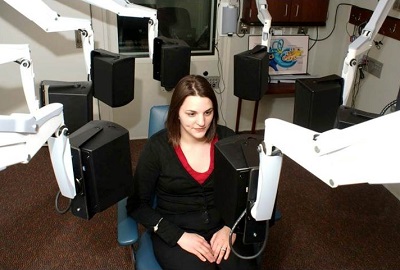
Figure 6. R-SPACE eight-speaker arrangement for study purposes.
Results
So how did they do? We looked at the dB SNR required to obtain 50% correct using the speech reception threshold (SRT). In this case, a lower number is better. We noted a lower dB SNR when the ipsilateral acoustic hearing was added to the implanted ear. In fact, it was about a 2-dB SNR improvement average across the English and Polish speaking groups. Statistically speaking, this was a highly significant finding.
You might be wondering how these patients would do if they only had the acoustic hearing. Some of them had near-normal hearing through 500 Hz. There are two things to take home from the data we obtained. One is that the implant alone did provide tremendous benefit; most of these individuals performed very poorly without the use of the implant. The second point is that the use of the hearing aid in the implanted ear provided considerable benefit for many of these individuals. For a very large percentage of these people, even though they are doing quite well, they are not even approaching performance levels that we would expect from normal-hearing individuals. It is important for us to recognize that. Even though we see very good performance in our patients, they are still showing a deficit in speech recognition in background noise. We did note that some of the Polish-speaking subjects did very well in their best aided EAS condition, approaching or even doing better than the normal-hearing individuals.
I also wanted to point out that this was not the first study to show that finding. Dunn et al. (2010) showed that in a spondee recognition task with the best aided EAS and bimodal arrangements, the subjects performed better in the best aided EAS condition than both the bimodal and the hybrid conditions. The hybrid electric-acoustic condition was only in the implanted ear. We are starting to see that patients with bilateral acoustic hearing are doing better in complex, diffuse and semi-diffuse noise fields.
In the +6 dB SNR condition, we observed, for both language groups, the same trend of a 10% improvement in performance when the subjects have both acoustic hearing ears. That was a statistically significant finding. We noted the same finding of a 10% to 12% improvement at +2 dB SNR when you are able to take advantage of both acoustic hearing ears. This also was a statistically significant finding.
Reverberation was a bit more difficult to measure because reflections are difficult to extract, but we did note a significant average improvement of six to seven percent for the individuals taking advantage of both the ipsilateral and contralateral acoustic hearing.
To summarize the Gifford and colleagues paper (2013), we found that preservation of acoustic hearing provided significant benefit for speech recognition in complex listening environments. To quantify, there was approximately a 2 dB improvement in the SNR, which translated to an improvement of anywhere between 6% and 12%, depending on the test condition (+6, +2 or reverberation).
What could be the underlying mechanism accounting for this improvement? Could it be the individual’s ability to use the ITD cues, as we have been hypothesizing? Patients tend to have hearing in the frequency region for which the ITDs are most prominent, which is under approximately 1500 Hz. Rather than hypothesize if the patients had preserved ITDs, we decided to measure them. We started with a simple 250 Hz pure tone. We fixed the level of the tone in both ears at 90 dB SPL; this correlated to between 10 and 40 dB SL for these individuals. We used a 2-down, 1-up tracking paradigm to find 70.7% correct on the psychometric function. These subjects were listening using only their acoustic hearing; they did not wear their implant. We used headphones over their native acoustic hearing. After hearing the sound in ear each individually, the task was to determine whether the sound moved from right to left or left to right. The subjects respond by pressing buttons on a response box. We showed the data for 6 of those individuals in the Ear and Hearing paper (Gifford et al., 2013), but we now have 12 subjects included to date, which I will include when talking about the data from that study.
We examined how much benefit the acoustic hearing in the implanted ear provided patients as a function of their ITD threshold in microseconds for a 250 Hz stimulus. Normal-hearing individuals have anywhere from a 30 to 50 microsecond threshold for a 250 Hz stimulus. The study subjects performed more poorly on average than normal-hearing individuals, although one person was close. They do have hearing loss, even in the low frequencies. More importantly, we are seeing a significant correlation between the ITD threshold and how much benefit can be obtained from the acoustic hearing in the implanted ear. This tells me that they have at least some preservation of binaural hearing cues or ITDs. Granted, this is not perfect, but they do have ITD thresholds. It is correlated with how much benefit they receive from the residual hearing in the implanted ear.
In the real world, we typically would encounter a maximum ITD of close to 800 microseconds, as long as speech was coming directly to the side of you. Is it the case that those who have the best preserved hearing also have the best ITD cues? The short answer is yes. There is a significant correlation between low-frequency pure-tone average in the implanted ear as a function of the ITD threshold. Those who have the best hearing with the lowest thresholds also tend to have the best ITD thresholds. People who have low frequency pure-tone averages of about 60 dB could have decent ITD thresholds or they could have very poor thresholds. Those who had the best preoperative hearing also had the best preserved hearing after surgery, which means that they had the best ITD thresholds and the greatest degree of hearing preservation benefit. That tells us that you have to implant people with residual hearing to start with, and you do have to preserve it after surgery. Based on this paper (Gifford et al., 2013) those patients will have the best outcomes.
Limitations
In the bimodal condition, we occluded the implanted ear. In real life, these patients use bilateral acoustic hearing with hearing aids. So when we take away that hearing for the research study, it is not a normal condition to which they are accustomed and that could have confounded the outcomes. Next, even though we had a large sample by these standards, it was still fairly small, especially for the ITD experiment. Clearly more investigation is needed in this area.
The Commodore Award
Gus Mueller: Vanderbilt University was named after Commodore Cornelius Vanderbilt whose sizable donation founded the university. In each Vanderbilt Audiology Journal Club, our presenter selects one article of particular significance to receive the Commodore Award. René, which article did you choose today?
René Gifford: Gunesh Rajan is a surgeon at the University of Western Australia in Perth, who has done a lot of research into hearing preservation with cochlear implants. The Commodore Award goes to Dr. Rajan’s triologic thesis from The Laryngoscope in 2011. He and his colleagues looked at the role of intratympanic steroid use during implant surgery and whether or not it improved hearing preservation outcomes. This was a prospective, interventional study, meaning that they provided an intervention and then evaluated the outcomes of that intervention.
Methods
They included 34 subjects, all of which presented with a cochlear implant and had measurable acoustic hearing prior to surgery. A number of patients received different implants. Nine of the patients were getting the MED-EL Flex EAS, or as we know it today, the Flex24, and 25 of the patients were getting the Flex SOFT. This is another electrode that is not yet have available in the U.S. It is a longer electrode than the Flex EAS. It was designed for people who have a small amount hearing to preserve, so you want a deeper insertion length. Twelve of the 25 patients received intratympanic steroids during surgery, and 13 did not.
In the control group, everyone received intravenous dexamethasone. That is standard procedure in most surgeries, if for no other reason than to help with nausea. They all were implanted with minimally traumatic surgical techniques and round window insertion. That is Dr. Rajan’s surgical philosophy. The interventional group also received all of that, but as soon as the patient was intubated, they received a transtympanic injection of methyl prednisone into the middle ear, which would allow for an infusion through the membranous round window and membranous portions of the middle ear in order to start getting into the patients’ system. Everything else, other than this transtympanic injection, was held constant.
The Flex SOFT is a longer electrode designed for up to 31.5 mm insertion. It is very similar to the H electrode, but it has a thinner apical edge. The Flex24, which was the Flex EAS, has a slightly shallower insertion depth.
The patients who had the best pre-operative hearing were implanted with the Flex EAS or the Flex 24. They found those who had poor hearing outside of these indications received the Flex SOFT. Then they classified patients on the basis of their postoperative hearing preservation. Level 1 included those who showed no statistical difference (less than 10 dB) between pre and postoperative hearing. Level 2 included those with partial hearing preservation, or between 10 and 30 dB loss of hearing. Level 3 were those who had greater than a 30 dB shift in hearing, or minimal hearing preservation, and Level 4 participants had a complete loss of hearing, which was labeled failure.
The preoperative audiograms for the patients implanted with the Flex SOFT looked more like traditional implant candidates, with moderate to severe and severe to profound hearing losses. The Flex24/Flex EAS recipients had anywhere from normal or mild hearing in the lows sloping precipitously to profound hearing loss.
Results
What did they find? All of the subjects received the transtympanic steroid injection. Of the Flex SOFT recipients, one half received the intratympanic injection (experimental group), and the other half did not (control). The hearing preservation rates were higher in the interventional groups when compared to the control group. Many more subjects were in hearing loss Level 1 and 2, and none of them were in the complete loss category.
Statistically speaking, they found that patients who had the round window insertion, the minimally traumatic surgery, the dexamethasone in their IV plus the transtympanic injection of methyl prednisone had greater preservation of hearing than those who did not have the additional steroid administration.
Limitations
What are some of the limitations of this study? Even though this was a prospective interventional study, they did not conduct it as a randomized clinical trial. That means that they did not randomize individuals into the intervention group or the nonintervention group. It was also a relatively small sample size. However, the results of this study, as well as the increasing number of animal studies that we have seen to date, are showing considerable preservation of acoustic hearing, and much more when there is additional steroid use.
Whenever the body faces trauma, it is going to activate an inflammatory response. Trauma includes minimally traumatic surgery, because you are still placing a foreign body into the cochlea. Each person’s inflammatory response is different. Some people are predisposed to more inflammation than others. The steroids act as an anti-inflammatory. If you can inhibit the inflammatory process, the thought is that you can have greater postoperative preservation of hearing.
Conclusions
Hearing preservation does lead to better performance, especially in complex listening environments. The degree of preserved hearing does impact the degree of EAS benefit. The use of intratympanic steroids does seem to be associated with better rates of hearing preservation. Patients who have the best hearing preservation also tend to have the best preserved binaural cues, as shown by the ITD thresholds, and the postoperative hearing in the implanted ear was most correlated with the best ITD thresholds.
There are still many questions to which we do not know the answers, such as, “How much hearing do we need to preserve?; Is there a minimum amount of hearing that one should have in order to see this benefit?; Do we need to amplify the entire bandwidth of the low-frequency hearing, or should we pay more attention to ultra-low frequency amplification?” We do not have prescriptive targets for 125 Hz. I am concerned with 125 Hz and 250 Hz in the laboratory, and we do not even have prescriptive fitting targets there.
Even if our patients show ITD thresholds, can they use them in the real world? Because we know that hearing aids and cochlear implants make use of automatic gain control (AGC) circuits, which we know disrupt interaural listening difference (ILD) cues, is it possible that some of these circuits, which are not synchronized, disrupt ITD cues as well? We did not touch on this, but what about the timing disruption between the electric hearing and the acoustic hearing, especially when it is being delivered to the same ear? There are so many different questions and so much fertile discovery that still needs to be made. For people who have two hearing aids and possibly two cochlear implants, there is a lot of work that still needs to be done in our clinical practice protocols and our research protocols as well.
Questions and Answers
Does your team recommend that the cochlear implant recipient use a hearing aid if there is preserved hearing in the implanted ear?
Yes, we do.
Do your patients then wear a separate hearing aid in the ear with the cochlear implant?
Yes, they do.
Do they ever discontinue acoustic hearing in the same ear with the cochlear implant?
Yes. I am funded by the National Institutes of Health (NIH), and I have funds where I can provide a patient with hearing aids for the implanted ear. Surprisingly, not every person takes me up on that, primarily because it also means that they have to spend extra time in the lab for a couple of days. But there are several that do elect to try hearing aids. Most patients fitted with an in-the-ear (ITE) hearing aid in the implanted ear have derived tremendous benefit from it. They wake up in the morning and put on two hearing aids and their cochlear implant sound processor. They find it beneficial enough that they are willing to deal with three devices.
Having said that, it is also important to note that I have at least a handful of patients who have been fitted with an ITE in the implanted ear and who have chosen to not wear that device full time. These are the patients, however, who have the greatest loss of hearing following surgery and tend to derive the least amount of benefit from it. I have at least two patients that showed significant benefit, but have decided that it is not worth the effort to wear two hearing aids and an implant processor on a daily basis.
We measure postoperative hearing in every implant patient at activation, and then we also check it one month later. Many of our patients still have residual blood and fluid in the middle ear that creates a conductive overlay. If our patients have any preserved hearing at all, we are going to follow that hearing consistently through the first year and then annually thereafter. We always are going to recommend that they consider a hearing aid in the implanted ear with residual hearing.
At the cochlear implant fitting, do you measure thresholds prior to device activation rather than after activation?
Yes, we do. When we see someone for the device activation, we take them back to the booth first. First we do otoscopy to make sure everything looks okay, and then we do the postoperative audiometric testing in that ear.
Most of the results I see of cochlear implant evaluations only show the CI levels.
That is probably true. I would say that there are not many centers that focus on the acoustic hearing in the implanted ear. It does not necessarily mean they are not measuring it, but they might not be recording it on the audiogram. There are some centers that are very research-based, but they do not always put their research outcomes on their clinical audiograms. That is a possibility.
Can we refer our patients to your clinic? What is the cost?
You can absolutely refer your patients to our clinic. There is no cost to enroll in the study, other than their time. My primary research project takes approximately 10 hours of listening. We tend to divide that over at least two days of listening because it is too much to do in one day. We have to see people at least twice because we need to get the ear mold impression, although they could get that in their home clinic. We need to get the hearing aid ordered, and then we tend to do the testing over a two-day period.
What ITE devices do you use?
I can tell you that I have tried many different devices over the years. I am not promoting any particular device, and several companies make quality ITEs. I have had good luck recently with the Phonak Virto Q ITE. It is on the new Quest platform and takes a size 13 battery. I like it because it has a lot of flexibility in the low frequencies; I have struggled with that with other devices, where I would only have one or two low-frequency bands to work with. I am finding that I am able to provide quite a bit of low-frequency audibility with the Virto for these patients.
Other than audiometric and speech perception testing, what other testing you use to verify hearing preservation?
We have been looking at spectral resolution pre and postoperatively with almost all of our patients now. Although I love the audiogram, I do not put a tremendous amount of stock in it because it is just a detection task. We know that a detection task is a lower-level task of sensory processing, and spectral resolution gives us more functional information. We look to see if we were we able to preserve the detection as well as the perception of the spectral resolution.
We have been doing spectral ripple testing, pre and postoperatively. Recently, we have been looking more at the possibility of cochlear dead regions pre and postoperatively as well. We have a paper coming out soon looking at the TEN test and the psychophysical tuning curve test from Alexander Sek and colleagues (2005). Obviously, this is not something that you can do in every clinic because it is time consuming. We are very fortunate to have research staff here at Vanderbilt where we can do all of these tests. With minimal extra effort and time, I think you will find that many patients are able to derive benefit from having amplification in the implanted ear.
References
Dunn, C. C., Perreau, A., Gantz, B. J., & Tyler, R. S. (2010). Benefits of localization and speech perception with multiple noise sources in listeners with a short-electrode cochlear implant. Journal of the American Academy of Audiology, 21(1), 44-51.
Gifford, R., H., Dorman, M. F., Skarzynski, H., Lorens, A., Polak, M., et al. (2007). Cochlear implantation with hearing preservation yields significant benefit for speech recognition in complex listening environments. Ear and Hearing, 34(4), 413-425.
Vinay, S. N., & Moore, B. C. (2007). Prevalence of dead regions in subjects with sensorineural hearing loss. Ear and Hearing, 28(2), 231-241.
Rader, T., Fastl, H., & Baumann, U. (2013). Speech perception with combined electric-acoustic stimulation and bilateral cochlear implants in a multisource noise field. Ear and Hearing, 34(3), 324-332.
Rajan, G. P., Kuthubutheen, J., Hedne, N., & Krishnaswamy, J. (2011). The role of preoperative, intratympanic glucocorticoids for hearing preservation in cochlear implantation: A prospective clinical study. The Laryngoscope, 122(1), 190-195. doi: 10.1002/lary.22142.
Sek, A., Alcantara, J., Moore, B. C., Kluk, K., Wicher, A. (2005). Development of a fast method for determining psychophysical tuning curves. International Journal of Audiology, 44(7), 408-420.
Cite this content as:
Gifford, R. (2013, August). Effects of hearing preservation for cochlear implant outcomes - Vanderbilt audiology’s journal club. AudiologyOnline, Article 12078. Retrieved from: https://www.audiologyonline.com


