Editor’s note: This text-based course is an edited transcript of a live webinar.
Learning Objectives
After this course learners will be able to:
- Describe the symptoms of chronic subjective dizziness.
- Describe the characteristics of the most common types of CSD.
- Describe the most common options for management of CSD.
Today, I am going to discuss Chronic Subjective Dizziness (CSD). As we find out more and more about diseases and disorders, sometimes their names change. The title of this presentation is Chronic Subjective Dizziness a.k.a. Persistent Postural Perceptual Dizziness (PPPD). Understand that this is an evolving concept.
Effects of Context and Anxiety on Behavior
Dr. Jeffrey Staab of Mayo Clinic, Rochester, has authored the research and the written material describing CSD and PPPD. The primary references for this talk can be seen in Figure 1, with a great deal of information drawn from the Staab (2012) paper and the Staab (2015) book chapter. My goal is to faithfully present this material to the best of my ability.

Figure 1. Primary reference sources.
Impairment can modify behavior, and behavior can change, depending on context. Let’s start by considering how we modify our behavior by the context of our experience. To illustrate this point, Dr. Staab asked the reader to imagine two people working in a warehouse. One of these workers is standing on the floor of the warehouse and the other is standing on a narrow catwalk, many feet above the warehouse floor. Both are moving back and forth, placing things on a high shelf. Even though the area where they’re moving has the same physical dimensions (it’s narrow), the worker on the catwalk moves more slowly, and turns more carefully than the worker standing the floor of the warehouse. Also, because of the penalties for a misstep, the worker on the catwalk raises up on their tiptoes slowly to place things on a high shelf, compared to the worker performing the same activity whose feet are firmly planted on the floor. The context of the physical activity can determine the real-world response to it.
In another example, Staab describes the behavior of a strain of anxious mice (yes, we have the ability to breed anxious mice). These mice were given the task of traversing a rotating beam. The investigators reported that the anxious mice slipped or paused more often in this task than the non-anxious mice. Most interestingly, the investigators reported that the behavior of these anxious mice could be “normalized” when they were treated with an antidepressant or an anxiolytic medication.
Patients with motion-provoked symptoms often manifest panic reactions. This occurs because in the course of compensation for a unilateral impairment, the brain has recognized that the peripheral vestibular system is sending to it a distorted representation of reality. In fact, the patient is sitting or standing still instead of rotating. Because of this, the brain reorders the hierarchy of the sensory inputs, placing a greater emphasis on the higher fidelity visual input. This means that vision becomes a sense used for orientation. As such, if the environment is in motion, then the patient will feel like they’re in motion as well. This means that patients may become unsteady in active visual environments.
This tells us that behavioral factors can play an important role in unraveling the source, or sources, of the patient’s dizziness. Dr. Staab has stated that this behavioral approach to the assessment of dizzy patients has reduced the number of patients without diagnoses from 25% to 2% at the Mayo Clinic in Rochester.
Most clinicians have had the experience of evaluating patients whose complaints were non-vertiginous, and where quantitative testing was entirely normal, but for whom the impact of dizziness was severe or profound. For those patients, we might have suggested that the cause was entirely extravestibular. It is significant to note that many years ago, the renowned otologist Cecil Hart, and neurologist David Drachman, estimated that 23% of the patients evaluated in their multi-dimensional dizziness clinic had either psychological or psychiatric disorders as the origin of the hyperventilation syndrome, demonstrated by anxious dizzy patients presenting to their clinic.
Evolution of Behavioral Approaches to Dizziness
Let’s take a step back and walk through the evolution of behavioral approaches to dizziness. In 1871, Karl Westphal, a famous German neurologist, coined the term agoraphobia. Agoraphobic patients described dizziness, spatial disorientation and anxiety that occurred when shopping in visually active public spaces. Thomas Brandt coined the term “phobic postural vertigo”, or PPV, to describe dizziness and unsteadiness that occurred in response to both environmental and social challenges.
In 1986, the investigators Brandt and Dieterich described patients with Phobic Postural Vertigo (PPV) as having obsessive-compulsive personalities, combined with either depression or anxiety. They noted that complete resolution of these complaints was uncommon. The common thread running through these early diagnoses of agoraphobia, psychogenic dizziness and phobic postural vertigo is that they were all non-vertiginous sensations. They occurred in wide open spaces where there was no physical reference point and they were all associated with psychiatric disorders, including anxiety and depression. The contemporary term to describe these sensations is Chronic Subjective Dizziness (CSD).
There are 3 primary factors that describe CSD:
- Persistent non-vertiginous dizziness that has occurred for 3 months or longer
- Hypersensitivity to either their motion, or motion of the visual surroundings
- Difficulty with precision visual tasks, like working at a computer
Dr. Staab has suggested that the term CSD replaces the older terms: Space and Motion Discomfort, Visual Vertigo and Phobic Postural Vertigo. Note that the investigators most recently have suggested that the term Persistent Postural Perceptual Dizziness replace the term Chronic Subjective Dizziness. It’s obvious that the describing and naming of this disorder is a dynamic process.
Epidemiology of CSD
It’s worth noting that anxiety in the general population occurs about 7% of the time and depression occurs in the general population about 5% of the time. When averaged across investigations, anxiety occurs approximately 27% of the time and depression occurs about 12% of the time, in the dizzy population. This means that anxiety appears approximately four times greater, and depression two times greater in the dizzy population compared to the general population.
CSD can occur across a lifespan, but appears to present most often when patients are in their 40s and 50s. Also, CSD occurs significantly more often in women than men. Further, CSD is associated with psychiatric comorbidities, like generalized anxiety disorder, panic and minor anxiety. Interestingly, and specific to the sex-linked preponderance of CSD for women, our group has reported that women volunteer greater somatic awareness, anxiety and depression, and self-reported dizziness handicap than men.
Symptoms and Diagnostic Criteria
Patients with CSD describe persistent non-vertiginous dizziness that may be associated with one or more of the following descriptors:
- Light-headedness
- Heavy-headedness
- Unsteadiness while ambulating which is not apparent to others
- Spinning sensation in the head with no motion of the visual field
- Rocking sensation that is not apparent to others
- Feeling that the floor is moving beneath their feet
- Disconnection from the environment
- Chronic hypersensitivity to self-motion
Locations where symptoms might be particularly bothersome would include grocery stores and shopping malls. The symptoms may wax and wane in severity during the day. Symptoms tend to be less severe in the morning. Symptoms are most severe when the patient is standing and walking, and less severe when sitting or laying down. There can be some positional symptoms as well, but CSD is not benign paroxysmal positional vertigo (BPPV). However, BPPV can lead to CSD. Again, this information comes from the publications by Staab and colleagues.
Behavioral factors can predispose patients to develop CSD. In particular, and according to Staab (2012), it is anxiety and introversion that predispose patients to develop CSD after a triggering event such as a vestibular neuritis. Anxiety over the symptoms produces a hyper-vigilant state. Both anxiety and depression add to the morbidity.
CSD occurs in patients without a history of neuro-otologic illness and patients with history of neuro-otologic illness (such as BPPV and/or vestibular neuritis), and in patients with neurological disorders, such as migraine and post-concussion syndrome.
The most common triggers for CSD include:
- Peripheral and central vestibular system disorders, e.g., vestibular neuritis, BPPV (25%)
- Panic attacks (especially for young patients), (15-20%)
- Migrainous vertigo (15-20%)
- Generalized anxiety disorder (GAD) (15%)
- Mild traumatic brain injury (10-15%)
- Dysautonomias (7%)
- Dysrhythmias (1-2%)
Topping the list are peripheral and central vestibular system impairments, panic attacks and migrainous vertigo.
CSD Types
Psychogenic CSD
The first type of CSD described by Staab is referred to as psychogenic CSD. These patients have no physical disorders but develop CSD as part of their general clinically significant anxiety disorder.
The following is a case history illustrating psychogenic CSD. The patient is a 21-year-old female with a history of panic attacks and previous psychiatric diagnosis of generalized anxiety disorder. The patient complains of unsteadiness when she walks. Further, she has a constant sensation of motion, commonly a rocking sensation, when she is sitting still. The sensation is like she’s on a boat, but she has not taken a recent voyage. She has had these continuous sensations for more than 6 months.
Otogenic CSD
The second type of CSD is referred to by Staab is otogenic CSD. In this case, there is a precipitating otogenic event. The otogenic event can be anything affecting the vestibular system. The most common otogenic events are vestibular neuritis, BPPV and migrainous vertigo.
This illustrative case history is that of a 40-year-old male who was in good health until one year ago when he had a two-day episode of vertigo that began while driving home from work. He was able to exit the highway safely and called his wife to pick him up and drive him home. The episode resulted in a trip to the emergency room and a diagnosis of the vestibular neuritis. The patient’s dizziness improved over a three-week period. Now, he is left with a constant dizziness that is aggravated when he scrolls text on a computer monitor or when he’s at a mall and there are people milling about.
Interactive CSD
The third type of CSD is referred to as interactive CSD. Patients with interactive CSD have a prior history of clinically significant anxiety, then experience an otogenic event, and then develop a worsening of the pre-existing anxiety that results in the symptoms of CSD.
The third illustrative case history is that of a 50-year-old female with a past medical history of gastroesophageal reflux disease, headache, anxiety and depression. She reports having repeated episodes of short-lasting, very intense vertigo. The duration of these episodes ranged from seconds to a minute, and were probably BPPV that occurred when she changed her head position. She is symptomatic when sitting or moving. She’s had the symptoms for more than 3 months.
These three types of CSD occur approximately one-third of the time each. There is recent data from Dr. Staab showing that the type of predominant behavioral disorder varies by the type of CSD. For example, Staab reports that panic disorder is the predominant psychiatric disorder found in psychogenic CSD. In comparison, lower levels of panic and minor anxiety are found in patients with a diagnosis of otogenic CSD. Lastly, generalized anxiety disorder is the predominant disorder seen in interactive CSD.
A key question is, “What factors predict those patients who will progress from acute vestibulopathy to CSD?” It appears that these patients have high resting levels of anxiety before the vertigo trigger occurs. Patients who have chronic vertigo tend to have enhanced somatic attention. They also catastrophize their symptoms. That is, the patient might think, “These symptoms are going to get worse and worse until I can’t take it anymore,” or, “This dizziness means I have a brain tumor.” Twenty percent of migraineurs also experience CSD. It has been recommended by Staab that treatment of these patients should focus on managing the migraine, the accompanying anxiety and the vestibular complaints.
CSD also occurs in patients with post-concussion syndrome, people with problems with memory and concentration, and people with insomnia. In addition to patients with migraine headache and head trauma, CSD has been reported for patients with dysautonomias. These are patients who have disorders affecting central neurovascular control. These patients become pre-syncopal and may lose consciousness when they change position where normal auto-regulation of blood pressure is impaired.
Theoretical Model for CSD
Attempts have been made to create a theoretical model for Chronic Subjective Dizziness. Staab and colleagues (2012) created a schematic for CSD. Their schematic indicates that CSD begins with a precipitating event.
The precipitating event could be, for example, an acute vestibular impairment, a head trauma or a panic attack. This event results in dizziness.
Acute adaptation then occurs. The reaction to the dizziness can be the automatic re-ordering of the sensory hierarchy that the brain uses for postural stability. In this case, the brain might switch from relying on vestibular system information to relying instead on sensory input from vision and somesthetic systems. The patient might adopt risky strategies to maintain their balance during their dizziness. Instead of a normal gait, the patient may stiffen and take short shuffling steps, which may place them at a greater risk for unsteadiness and falls. Lastly, the patient’s hypervigilance over the environment may require more attentional resources than normal and also may be manifested as vision dependence.
Once the acute event resolves, recovery occurs, where the patient may return to their pre-morbid state. The amount of time that is required for recovery is dependent on a number of factors, including: whether the event produced a permanent injury to the vestibular system (or whether recovery actually occurred), the age of the patient, their activity level, the state of contralateral vestibular system, whether they have been placed on long-term vestibular suppressant medications, and the status of the CNS.
Predisposing factors. Under normal circumstances, the patient with a unilateral vestibular impairment eventually behaves normally. Problems begin when the patient has predisposing or preexisting factors, such as clinically significant anxiety and an introverted temperament. These factors can increase the likelihood that postural and behavioral responses to the acute dizziness will be magnified. These responses include anxiety, vision dependence and somatosense dependence.
These responses feed back on themselves, creating a perpetuating loop, increasing anxiety and vision dependence. This can result in panic episodes and postural destabilization that occurs because of exposure to active visual environments.
Predictors of Poor Outcomes
Predictors of those patients who will transition into CSD are those:
- With high pre-morbid anxiety
- Who are hyper-vigilant over their dizziness symptoms
- Who catastrophize over possible outcomes from their dizziness
These are patients who failed to “un-adapt” after either compensation has taken place for unilateral peripheral vestibular system impairments, or after function has returned to the formerly impaired end organ. These are patients who are destabilized by their own motion, or by motion of the environment, and become symptomatic in situations that require complex visual demands.
The concept that an event occurs and triggers a cascade of reactions to it, some of which are maladaptive, may sound familiar. Many of you may be familiar with the neurophysiological theory of tinnitus as described by Jastreboff and Hazell (2004). This theory describes how tinnitus can become bothersome, disabling or handicapping.
The general idea is that for most individuals, tinnitus remains at a subconscious level. It is there, but it is not perceived. For some, it is perceived, but the perception of tinnitus remains within the auditory pathway. It is when tinnitus is perceived, and there is an emotional reaction to it (e.g., fear, anxiety and depression, where the patient may catastrophize over it), patients are unable to ignore the signal that once was at a preconscious level. The negative feedback results in an activation of the autonomic and limbic systems. This is when tinnitus becomes troublesome for patients and they end up seeking our services.
Diagnosis of CSD
It is the role of the audiologist to conduct the quantitative assessment of the vestibular system. The finding of abnormal test results might assist in the identification of patients with otogenic or interactive CSD. A second role is to suggest to the referral sources that they consider the possibility of CSD in their differential diagnosis for the patient. Lastly, we can help patients a great deal by teaching them how the vestibular system works when everything is functioning normally, and how the vestibular system doesn’t work when it is malfunctioning. For most patients, a professional taking time to sit down and explain why they feel so sick can be empowering, and a treatment of sorts. A thorough case history can help begin the sorting-out process of those patients who have true vertigo, dizziness, light-headedness and unsteadiness.
A number of problems, over and above peripheral vestibular system impairments, can result in CSD. The most frequent problems include vestibular migraine, panic and generalized anxiety. The other components of the assessment include: measures of self-report anxiety and depression, and the conventional electroneurodiagnostic assessment of the vestibular system, including Video Head Impulse Test (vHIT), VNG, Vestibular Evoked Myogenic Potentials (VEMP), rotary chair testing and posturographic measures. Signs of significant anxiety and/or depression might represent bits of evidence that will help sort things out once the quantitative assessment has been completed.
Dr. Staab suggests that the clinician be aware of three important questions in their assessment of the patient:
1.) Does the patient have an active peripheral or central vestibular system impairment?
With this question, we are looking for semi-objective evidence that the patient has an organic disease as a cause of the dizziness.
2.) Does the impairment explain completely the patient’s symptoms?
In other words, is the patient’s reaction to the disorder greater than one would normally expect or anticipate?
3.) Has the patient reported, either in interview or by questionnaire, distress or changes in their behavior as a response to the impairment.
Hospital Anxiety and Depression Scale (HADS)
The device we use for assessing whether the patient demonstrates abnormal self-reported anxiety and/or depression is called the Hospital Anxiety and Depression Scale, or HADS (Zigmund & Snaith, 1983). High scores on the HADS correlate positively with high scores on the Dizziness Handicapped Inventory (DHI). The HADS is comprised of 14 items and 2 subscales. There are seven items in the anxiety subscale and seven items in the depression subscale. Each item is scored from 0 – 3 points, with a maximum score of 21 for each subscale. Abnormal performance begins when subscale scores are equal to or greater than 11 points.
For example, question 1 on the HAD is an anxiety item. It reads as follows:
I feel tense and ‘wound up’
a. most of the time
b. a lot of the time
c. from time to time, occasionally
d. not at all
Three points are scored for answer A, 2 points for answer B, 3 points for C and 0 points for D.
Question 2 on the HADS is a depression item. It reads:
I still enjoy the things I used to enjoy:
a. definitely as much
b. not quite so much
c. only a little
d. hardly at all
Scoring is 0 points for A, 1 point for B, 2 points for C, and 3 points for D.
The correlations are moderately positive for the HADS anxiety and depression subscales, and a total score on the DHI. That is, as total DHI scores increase, so does both self-reported anxiety and depression (per unpublished data from Kurre et al. and Piker et al.).
Dizziness Handicap Inventory (DHI)
The Dizziness Handicap Inventory was first published by Jacobson and Newman in 1990. There are 25 items evaluating self-reported dizziness handicap. There are three subscales: functional, emotional, and physical. Each item consists of a question that must be answered by the patient by saying “yes,” “no,” or “sometimes.” A yes response is scored 4 points, a sometimes response is scored 2 points and a no response is scored 0 points. The maximum score on the DHI is 100 points representing maximum self-report dizziness handicap. The lowest score is 0 points representing no self-report dizziness handicap. A score equal to or greater than 46 points equals a severe self-reported dizziness handicap.
A few representative items taken from the DHI are as follows:
- P1. Does looking up increase your problem?
- E2. Because of your problem do you feel frustrated?
- F3. Because of your problem do you restrict your travel for business or recreation?
- P4. Does walking down the aisle of a supermarket increase your problem?
Notice that the words “dizziness,” “vertigo” and “unsteadiness” have been replaced with the words “your problem,” in an effort to make the device more usable for patients who do not have true vertigo.
Electroneurodiagnostic Tests
Neurodiagnostic tests such as ENG/VNG, VEMP, and rotary chair testing have been extremely helpful in identifying those patients with either otogenic or interactive CSD. Computerized dynamic posturography (CDP) has also been very helpful in demonstrating vision dependence in this cohort of CSD patients.
CDP testing. Let’s talk a little bit about posturographic testing and anxiety and depression. NeuroCom developed a device consisting of a platform that was capable of sensing forward and backward sway and a surround that consisted of an image of the horizon. A patient stands on the device wearing a harness that is attached to a frame so if the patient falls, they will not be hurt. The patient is asked to remain as stable as possible. The platform and the surround can be made to move with the patient, or not. The patient may be asked to close their eyes or not. There are 6 conditions where patients must do their best to remain steady:
Condition 1: both the platform and surround are stable.
Condition 2: the platform and surround are stable but the patient must close their eyes.
Condition 3: the patient’s eyes are open but the surround will sway with the patient if the patient sways.
Condition 4: the surround is stable but the platform will sway with the patient if the patient sways.
Condition 5: the patient’s eyes are closed and the platform will sway with the patient.
Condition 6: the patient regains their vision but the platform and the surround will sway with the patient.
This test is used to help investigators determine to what extent patients rely on vision for postural stability. The test is also used to help us understand whether compromised somesthesia and vestibular function is a source of postural instability.
Investigators have reported that patients with clinically significant anxiety may demonstrate evidence of subtle vestibular system impairments. In fact, it has been reported that patients with panic disorder and no vestibular impairment may be quite unsteady on even the first condition of the Sensory Organization Test. Normally, patients are most steady on the conditions where neither the platform nor the surround are sway referenced. These patients also were more unsteady in conditions where their eyes were open (compared to closed) when both of the platform and surround were sway referenced. This behavior was first described by Cevette and referred to as “an incongruous pattern” and was initially attributed to malingering. Staab has taken issue with that interpretation, and feels that this pattern of response is characteristic of what one would expect to see from a patient who is both vision and platform dependent for postural stability. These are patients who rely more on vision and the somatosenses for balance than the vestibular system.
Cevette and colleagues in 1995 show sensory organization results from a patient that may have had CSD. This patient was more unsteady on even the easiest first condition compared to the most difficult 5th and 6th conditions. Also, for this patient, when the eyes were open in either the surround or platform or sway reference conditions, the patient became very unsteady. The patient was unsteady in part due to dependence on both vision and the somatosenses (rather than vestibular function) to remain upright.
Treatment of CSD
According to Dr. Staab, there are three primary routes of management for CSD: medical/pharmacological, counseling/psychotherapy and physical therapy (including vestibular rehabilitative therapy).
Medication
Staab has reported that pharmacological research has mostly involved open label trials where both the researchers and the participants know which treatment is being administered. The medications that seem to be associated with the greatest success are the selective serotonin re-uptake inhibitors (SSRIs) like Zoloft and Prozac, or the serotonin-norepinephrine reuptake inhibitors, like Effexor and Cymbalta. Both medications produced greater than 50% reduction symptoms for 60 to 80% of patients that completed 8 to 12 weeks of treatment.
The Staab paper from 2012 includes a table that provides a great summary of the pharmacological treatment of CSD. The lead investigator reported that the subject dropout rate for the drug studies was 20% of the subjects because of the side effects. The dosage was one quarter to one half the initial dose to treat major depression. This dose was titrated to achieve a level consistent with that used for treatment of depression. Eight to 12 weeks were required for patients to notice the effects.
Vestibular Rehabilitation Therapy (VRT)
VRT has been used for patients with CSD and the success rate is about the same as for pharmacological therapy. VRT promotes compensation in the case where there is quantitative evidence of a peripheral or central vestibular system impairment. Further, Staab (2012) reports that where habituation exercises are being done, they must be done less aggressively in the beginning, or patients may become too symptomatic and will abandon therapy altogether. Accordingly, they may need many breaks. It may take three to six months before patients experience the positive effects.
Cognitive Behavioral Therapy (CBT)
The last type of therapy is cognitive behavioral therapy, or simply CBT. Staab (2012) references Edelman and colleagues (2012). They examined the effect of CBT on two groups: one group was on a waiting list for treatment, and the second was a treatment group. The authors reported that treatment had a medium effect for reducing dizziness, and failed to demonstrate an effect on anxiety or depression.
Three Case Studies
To conclude this presentation, we will analyze three specific case studies. These patients exhibit the typical symptoms and hallmarks of psychogenic CSD, otogenic CSD and interactive CSD.
Psychogenic CSD
The first case is a 28-year-old female with a one-year history of continuous non-vertiginous dizziness. The patient describes the sensation as rocking side-to-side or front-to-back. She compares this feeling as being similar to sitting on a boat in choppy water. She can’t define the date of onset, but she is certain it has been occurring for at least six months. She’s had no frank spells of true spinning vertigo, but has constant unsteadiness when sitting or walking. She carries a previous diagnosis of generalized anxiety disorder (GAD) and is taking Nortriptyline and Buspar. She also has a history of panic disorder.
Test results are included in your handout and I will summarize them here. Gaze testing is absolutely normal. Saccade testing shows normal saccade velocities and accuracy and latency. Pursuit testing shows normal pursuit gains from 0.2 Hz to 0.7 Hz. Optokinetic testing is also bilaterally symmetrical and normal. Spontaneous nystagmus test is also normal. Monothermal warm caloric test yielded a 5% asymmetry, which is within the normal range. We use a cutoff of 10%. Rotational testing shows normal VOR phase, gain and symmetry and also normal VOR suppression. cVEMP testing also is bilaterally normal, both the amplitude and latencies. The Sensory Organization Test of posturography is also normal.
To summarize, all of the semi-objective electroneurodiagnostic test results are normal.
At this point, a clinician who did not know better might have written a report saying that this was a normal examination. However, there are other assessments that I have not presented yet including self-report assessments of dizziness handicap, anxiety and depression. The results of these qualitative assessments showed the patient has significant self-reported anxiety and depression. Further, they rated their self-report dizziness handicap as severe. With a previous history of anxiety, depression and panic, this patient most likely has psychogenic CSD.
Otogenic CSD
The next case illustrates a typical presentation of a patient who has otogenic CSD. The patient reports a 9-month history of continuous non-vertiginous dizziness. The dizziness began approximately one month following a day-long episode of severe true vertigo and was associated with nausea and vomiting. Now, the patient feels unsteady during ambulation.
The DHI score of 70 out of 100 shows the patient to have severe, self-reported dizziness disability handicap. The results of the HADS show the patient to have significant self-reported anxiety, with a score of 15 out of 21 on the anxiety subscale. The HADS depression subscale score of 7 out of 21 is within the normal range. Remember, significant is any amount over 11 points for each of the sub-scales.
In your handout you can review the tracings and recordings for the various testing that was performed, and I will summarize them here. The gaze test is normal. Saccade subsystem testing showed normal saccade velocity, accuracy, and latency. Pursuit testing was also normal. Optokinetic nystagmus testing was normal and symmetrical. There was no evidence of spontaneous nystagmus. However, in this case, bithermal caloric testing revealed that the patient had no caloric response to warm or cool water or even ice water irrigations on the left side. Rotational testing revealed reduced low frequency vestibular ocular reflex (VOR) gains and these were associated with the predicted low frequency phase impairments. VOR suppression was normal, with no evidence of central vestibular impairment.
This is a case where the patient did not have a previous history of anxiety or depression. The patient sustained an otogenic event and now is left with symptoms that are attributable to the patient’s anxiety reaction to the initial episode.
Interactive CSD
The third case describes a patient who we feel most likely had interactive chronic subjective dizziness. This is a patient that we reported some years ago in a paper published in the Journal of the American Academy of Audiology (McCaslin, Jacobson, Burrows, Littlefield, & Haynes, 2010).
The patient was a 41-year-old female helicopter mechanic who worked in high noise levels. The patient complained of ear pain and fullness that occurred daily and was precipitated by loud sounds. This was a big problem for a person working around helicopters. The loud sounds were causing nausea and unsteadiness. Further, she complained of hearing loss in her right ear and numbness on the right side of her face. She also complained of frequent headaches. The patient’s medication history included, among other medications, an antidepressant and anti-nausea medication.
The patient had a hearing test that showed a right moderate mixed hearing loss and a left mild to moderate sensorineural hearing loss. Word recognition ability was good bilaterally. Stapedial reflexes were present bilaterally, which was at odds with the right-sided conductive component.
Neurotology reported their impression was that the patient had peripheral vertigo and bilateral low frequency sensorineural hearing loss. Neurotology sent the patient for vestibular function tests and at the same time initiated a neurology consult because of the headache history.
On the vestibular assessment, the patient scored 14 out of 100 points on the DHI, which was normal, indicating no self-report handicap. The patient scored 7 out of 21 points on the anxiety sub-scale of the HADS, and 11 out of 21 points on the depression sub-scale of the HADS. The latter score represented evidence of clinically significant depression. Bithermal caloric tests were normal. There was only a 3% unilateral weakness on the bithermal test. Rotary chair test results were normal as well.
Here is where things began to get interesting. The patient showed a normal cVEMP examination on the left side, but showed an abnormally reduced cVEMP threshold on the right side. The threshold was 65 dB nHL on the right side for the 500 Hz tone burst stimulus. The threshold was greater than 70 dB nHL on the left side. A normal threshold is in the range of 80 – 90 dBnHL.
Because the patient complained of a noise-induced vertigo, we conducted both a Tullio test and a Valsalva test. The Tullio test was the presentation of a steady 100 dB HL, 500 Hz tone to the right ear. That stimulus evoked a down-beating nystagmus, suggesting that the right anterior semicircular canal was being activated. The Valsalva test also evoked a down-beating nystagmus.
We summarized that the evidence we collected suggested the possibility of a right-sided anterior semicircular canal dehiscence (SCD). We recommended that the SCD be included in the differential diagnosis for this patient. As a result of our findings, the patient underwent a high resolution CT scan which confirmed that the patient had an anterior semicircular canal dehiscence on the right side.
The patient was referred to neurology for a consultation because of headaches. Neurology diagnosed the patient with chronic daily headache with right otalgia. They requested a repeat MRI and a lab test for Lyme Disease, which was normal. The patient was seen by neurotology for a second time because of her symptoms as they were persistent. For that reason, the patient wanted to proceed with surgery to correct the defect.
Accordingly, the patient underwent a right middle cranial fossa approach to repair the dehiscent anterior canal. The canal was plugged with bone wax, and the defect was covered with a bone graft and was secured with bone cement.
Postoperatively, the patient underwent a second high resolution CT scan and a second balance function assessment. The result of the high resolution CT scan verified the dehiscence was indeed repaired.
This slide shows the pre-post operative audiograms (Figure 2). With the exception of 250 Hz where thresholds worsened post-operatively and 4000 Hz where thresholds improved post-operatively, the thresholds were essentially stable.
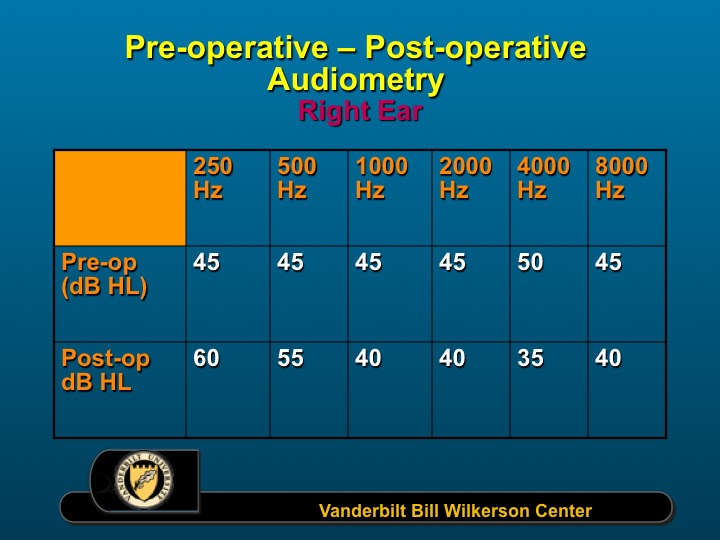
Figure 2. Pre-post op audiograms.
VNG Examination
At the post-operative balance function assessment, the patient was complaining of severe pain and a constant unsteadiness. When we saw her for a post-op examination, she was taking many more medications for anxiety and depression than she was before. Saccade testing and ocular motility in general was normal postoperatively. Caloric testing remained normal, as did rotational testing. Recall that cVEMP test preoperatively showed a reduced threshold for the cVEMP on the right side, and a normal threshold on the left side. Postoperatively, the threshold on the right side was now 85 dB nHL instead of 65 dB nHL.
The surgery was successful. However, there were some very large differences, pre-op to post-op in measures of self-reported dizziness handicap and self-reported anxiety and depression. Post-operatively, the patient demonstrated severe self-reported dizziness handicap (score = 68/100) and almost a maximum (21 points) on both the anxiety and depression sub-scales of the HADS, where there was only a significant depression pre-operatively.
Post-operatively, the vertigo changed from episodic to a continuous sensation of instability. The patient was discharged to physical therapy on the third postoperative day. The patient regressed from being ambulatory to using a walker, and then finally, a wheelchair. The patient began having pseudoseizures and for that reason was referred to psychiatry.
Post-operatively, the patient was diagnosed with interactive CSD and acute adjustment disorder (AD). The latter is a mental illness that is characterized by an abnormal behavioral response to an identifiable stressor or set of stressors. The department of neurology is treating the patient with anxiolytic and antidepressant medications, cognitive behavioral therapy and vestibular rehabilitation therapy.
To summarize this case, the patient began with a right-sided SCD and evidence of clinically significant depression. The dehiscence was repaired, which was the otogenic event, and now the patient has significant self-reported dizziness handicap, anxiety and depression post-operatively. This patient evolved from having SCD to interactive CSD.
Conclusion
We discuss CSD as though it’s a stand-alone diagnosis, and it can be. However, most of the time, CSD can be a comorbidity for a number of vestibular system disorders, including positional vertigo, vestibular neuritis, Meniere’s syndrome and migrainous vertigo.
In this regard, Eggers and colleagues (2007) described 88 consecutive patients referred to a tertiary clinic for assessment of vestibular migraine. Fifty-seven percent had at least one comorbidity. Patients with otologic comorbidities had increases in the presentation of handicap, anxiety and depression.
Neff and colleagues (2012) evaluated 147 consecutive patients meeting the criteria for Meniere’s Disease alone, vestibular migraine alone or both Meniere’s and vestibular migraine together. Vestibular migraine patients more often were female, younger, and with vertigo lasting seconds to days. Meniere’s Disease patients were more often male, older, and with vertigo lasting hours. In patients with either vestibular migraine or Meniere’s Disease, CSD was observed almost always (91% of the time) in the vestibular migraine group, not the Meniere’s group.
In summary, in the presence of CSD it is important for clinicians to consider the possibility of multiple coexisting vestibular diagnoses instead of just one. I want to thank Dr. Jeffrey Staab and his collaborators for their pioneering research in behavioral factors in dizziness.
References
Cevette M.J., Puetz B., Marion M.S., Wertz M.L., & Muenter M.D. (1995). Aphysiologic performance on dynamic posturography. Otolaryngol. Head Neck Surg., 112(6), 676–688.
Edelman. S., Mahoney, A.E., & Cremer, P.D. (2012). Cognitive behavior therapy for chronic subjective dizziness: a randomized, controlled trial. Am J Otolaryngol., 33(4),395-401. doi:10.1016/j.amjoto.2011.10.009.
Eggers, S.D. (2007). Migraine-related vertigo: diagnosis and treatment. Curr Pain Headache Rep, 11, 217-226.
Eggers, S.D., Neff, B.A., Shepard, N.T., & Staab, J.P. (2014). Comorbidities in vestibular migraine. J Vestib Res, 24(5-6), 387-395.
Furman, J.M., Balaban, C.D., Jacob, R.G., & Marcus, D.A. et al. (2005). Migraine – anxiety related dizziness (MARD): a new disorder? J Neurol Neurosurg Psychiatry 76, 1-8. doi:10.1136/jnnp.2004.048926.
Jacobson, G.P., & Newman, C.W. (1990). The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg., 116(4),424-7.
Jastreboff, P.J., & Hazell, J.W.P. (2004). Tinnitus retraining therapy. New York: Cambridge University Press.
McCaslin, D.L., Jacobson, G.P., Burrows, H.L., Littlefield, P., & Haynes, D.S. (2010). Transforming superior canal dehiscence to chronic subjective dizziness: from SCD to CSD. JAAA, 21(5), 293 – 300. doi: 10.3766/jaaa.21.5.2.
Neff, B.A., Staab, J.P., Eggers, S.D., Carlson, M.L., Schmitt, W.R., Van Abel, K.M....Shepard, N.T. (2012). Auditory and vestibular symptoms and chronic subjective dizziness in patients with Meniere’s disease, vestibular migraine and Meniere’s disease with concomitant vestibular migraine. Otol Neurotol 33(7), 1235-1244. doi: 10.1097/MAO.0b013e31825d644a.
Piker, E.G., Jacobson, G.P., McCaslin, D.L., & Grantham, S.L. (2008). Psychological comorbidities and their relationship to self-reported handicap in samples of dizzy patients. JAAA, 19, 337-347.
Ruckenstein, M.J., & Staab, J.P. (2009). Chronic subjective dizziness. Otolaryngol Clin N Am, 42, 71-77.
Staab, J.P. (2012). Chronic subjective dizziness. Continuum Lifelong Learning Neurol, 18 (5), 1118-1141.
Staab, J.P. (2013). Behavioural neuro-otology. In A.M. Bronstein (Ed), Oxford textbook of vertigo and imbalance (pp. 333-346). NY: Oxford University Press.
Staab, J.P. (2014). The influence of anxiety on ocular motor control and gaze. Current Opinion Neurology, 27(1), 118-124.
Staab, J.P. (2015). Behavioral factors in dizziness and vertigo. In G.P. Jacobson & N.T. Shepard (Eds), Balance function assessment and management (pp. 729-751). San Diego: Plural Publishing.
Zigmund, A.S., & Snaith, R.P. (1983). The hospital anxiety and depression scale. Acta Psychiatr Scand, 67(6), 361-70.
Further references may be available in the handout.
Citation
Jacobson, G. (2016, June). Chronic subjective dizziness (or persistent, postural-perceptual dizziness). AudiologyOnline, Article 17345. Retrieved from www.audiologyonline.com