Gallaudet University
Audiology Online Contributing Editor
Linda Herzberger-Kimball, M.S.
Kaiser Permanente
Denver
Shannon Burns, M.A., CCC-A
Phoenix Children's Hospital
Shay Darnell Balew, M.A., CCC-A
Phoenix Children's Hospital
Abstract
The auditory brainstem response (ABR) test is not widely utilized as a neurological assessment instrument because of superior imaging procedures currently available. The general purpose of this ongoing research is to develop an ABR test protocol that improves sensitivity to small vestibular schwannomas and subtle neurological disease. The specific goal of this study was to determine the degree to which wave V latency shifts with rapid stimulus repetition rates in normal subjects, and then apply that data to a test case. Currently, the standard criteria for tumor diagnosis using ABR are delayed wave V and prolonged interpeak latencies. The notion of using a rapid stimulus repetition rate as a diagnostic feature was proposed in the late 1970s (Don, Allen, & Starr, 1977; Stockard, Stockard, & Sharbrough, 1978), given the rationale that the minimal neural recovery time imposed by this technique significantly delays wave V latency when the auditory nerve is compromised. Burkard (1994) made the same observation when presenting very rapid stimulus rates. Nonetheless, the use of rapid stimulus rate was never adopted as standard diagnostic protocol in neuro-diagnostic assessment. The current study was conducted on normal subjects to determine if ABR waveform characteristics would be altered by a change in click stimulus rate. Stimulus rates of 31.1, 45.1 and 61.1/sec were used to evoke ABR waveforms from 20 otologically normal subjects. Wave V latencies were statistically compared between groups; wave V latency shifted predictably with each stimulus rate increase. A 50% increase in stimulus repetition rate over the baseline rate of 31/sec resulted in a predictable shift in Wave V latency by 0.094 msec. Analysis of these findings suggest that using two standard deviations (SD = 0.18 msec) from the mean latency of wave V at 61.1/sec (5.89 msec) would allow for normal variability in latency while identifying subtle neurological defect. Therefore, establishing Wave V latency at 6.25 msec as the lower limit for referral would be sufficiently sensitive to small vestibular schwannomas. This procedure accurately identified a patient in the present study with a small vestibular schwannoma when standard clinical protocol for ABR testing did not.
Background
Interest in electrical activity of the central nervous system certainly predates Hans Berger's first unpublished reports of the electroencephalogram (EEG) in 1924 (Jung & Berger, 1979), although this landmark discovery introduced a new era of neurodiagnostics. The medical application of the signal averaging computer was realized in the mid 1960s, enabling dissection of the EEG into its component and contributory parts. The EEG represents all body electricity, with only a percent or less contributed by the auditory nerve and auditory brainstem. The signal averaging computer eliminates, or 'averages out', the 99% of the EEG that is not auditorily generated and produces an auditory-only record, the ABR. The procedure is efficient, reliable and inexpensive. Furthermore, the ABR test is reported to have as high as 98% sensitivity in identifying large tumors of the auditory nerve and brainstem (Barrs, Brackmann, Olson, & House, 1985; Zappia, O'Connor, Wiet, & Dinces, 1997). Detection of tumors of only a few millimeters in diameter requires a more sensitive ABR procedure than is conventionally used.
The ABR test procedure has undergone relatively minor modifications since it was described in the late 1960s and early 1970s. By contrast, radiological imaging of the auditory nervous system has advanced from the 'blur' of early generation CT scans to the photographic-quality MRI. In fact, ABR testing as a neurological assessment instrument is often bypassed in favor of the MRI. Although the MRI is more effective at identifying smaller tumors, the cost of such procedures is typically significantly greater than the ABR screening (Robinette, Bauch, Olsen & Cevette, 2000; Cheng, Smith & Tan, 2003). Adding a simple procedure to the standard ABR routine which would make the test more clinically sensitive to neurological disorders would be an important achievement. Several studies describe methods of improving ABR sensitivity to small VIIIth nerve tumors. Bauch et al. (1996) reported that a combination of interaural wave V latency differences and I-V interpeak latency (IPL) data improved the sensitivity of standard ABR protocol to detect tumors of less than 1.0 cm. These techniques are not new, with interaural wave V latency differences first suggested by Selters and Brackman (1977) as a sensitive indicator of small acoustic tumors. Bauch et al. (1996) found 92% sensitivity to all tumors with 88% specificity. However, greatest sensitivity to small (
There are very few reports of ABR sensitivity of better than 80% to 1.0 cm size tumor or smaller. Perhaps more common is a report by Schmidt et al. (2001) who found in a dozen patients with tumors of less than 1.0 cm where 58% of cases were detected by ABR. As noted by Schmidt, et al. (2001) finding tumors of such a small size, which are entirely intracanalicular, is important for improved surgical outcomes in cases where hearing may be preserved. The methods in each of the previous studies were conservative in defining I-V IPLs and wave V absolute latencies as abnormal when they varied by 0.2 ms from the norms. Interaural wave V latency differences were not considered diagnostically significant by Schmidt, et al. (2001). Contralateral recordings have been reported to be abnormal with normal ipsilateral recordings when the vestibular schwannoma was too small to be detected by MRI (Coelho & Prasher, 1990).
The notion of using a rapid stimulus repetition rate as a diagnostic feature was proposed in the late 1970s (Don, Allen, & Starr, 1977; Daly, Roeser, Aung, & Daly, 1977; Stockard, Stockard, & Sharbrough, 1978), with the rationale that the minimal neural recovery time imposed by this technique would delay wave V latency due to an already pathologically stressed auditory nervous system. Lightfoot (1992) applied this concept to assessment of patients with vestibular schwannomas and reported a sensitivity of 100% and specificity of 97.9% when an 88/sec stimulus rate was used to screen for tumors. He reported that subject sex and stimulus sensation level did not adversely affect these test measures. However, Lightfoot (1992) cautions that in general, age of the subject was not a factor in latency shifts; however, results from subjects with "advanced age" should be interpreted with caution due to previously reported abnormal latency shifts . Typically for a rate increase from 7.7/s to 57.7/s, wave V latency increases of less than 0.6-0.8 msec have been reported in normal young adult listeners (Hood, 1998).
Burkard and Hecox (1983) also described changes in ABR morphology as a function of stimulus rate. For stimulus rate increases, changes included decreasing amplitude, increasing wave V latency, and prolonged I-V interpeak latency. Later, Burkard (1994) provided data on rate effects on the ABR with maximum length sequence (MLS) paradigm which involved pseudo-random pulse trains allowing for extremely high stimulus rates (500 Hz). Burkard and Sims (2004) showed that latency increase was nearly linear with stimulus rate increases between 25 and 75 stimuli per second and was non-linear with rates exceeding 75/sec. The overall Wave V latency shift reported by Burkard and Sims (2004) was 1.3 ms when the rate increased from 11 to 500/sec. Step increases from 25 to 50/sec and 50 to 75/sec were approximately 0.20 ms per each rate increase. Conventional methods of ABR acquisition do not allow for stimulus rates greater than 100/sec, assuming a 10 ms duration window. Therefore, the MLS paradigm is not applicable for standard, commercially-available ABR instrumentation.
A study by Tanaka, Komatsuzaki, and Hentona (1996) reported the effects of stimulus rate on the ABR in patients with identified vestibular schwannomas. ABRs were recorded using stimulus rates of 9.5, 20, 40, and 90/sec at 90 dBnHL from 40 ears with confirmed vestibular schwannomas. Results demonstrated a significantly larger interpeak latency (IPL) of Wave I-V and interaural latency difference of Wave V in vestibular schwannoma patients compared to the control group for all stimulus rates. Six patients with small, intermeatal vestibular schwannomas exhibited normal ABR results when recorded at 9.5 Hz; however, when the stimulus rate was increased to 90 Hz, five of the six subjects had abnormal I-V IPLs or a significant change from the baseline measure. The authors of this study concluded that incorporating a high-stimulus rate recording can increase the sensitivity of the ABR in the detection of small, intermeatal tumors.
The purpose of this study was to examine ABRs in otologically normal subjects at 31.1/s, 45.1/s, and 61.1/s rates. These rates were selected for several reasons:
- The rates fall along the linear continuum of rate increase and wave V latency shift as identified by Burkard and Sims (2004);
- The rates are available on all standard evoked potentials instrumentation; and
- The fastest rate (61.1/s) is well below the maximum allowable rate using a 10 ms window,
- increased sensitivity to multiple sclerosis reported by (Jacobson et al., 1987) and Robinson and Rudge (1977).
Procedure
Subjects
Twenty subjects with normal audiometric hearing and no history of otologic disorders were evaluated. The age range was 19 - 26 years (mean = 23.4 years). There were seventeen female and three male subjects.
Equipment
A Biologic Traveler auditory evoked potentials unit was used to record data. Stimulus intensity was calibrated in dBnHL; amplitude and stimulus rate were calibrated using an HP dual trace oscilloscope at the beginning and end of each test session. Stimuli were delivered via TDH-49 earphones in MX-41/AR cushions. Signals consisted of 75 dBnHL 0.1 ms rectangular pulse trains presented at 31.1, 45.1 and 61.1 stimuli per second. Psychoacoustic judgments of stimulus loudness as a function of rate change were made by 5 of the subjects to determine whether a change in signal inter-stimulus interval (ISI) might produce a perceptible loudness variant. The various rates were judged to be equivalent at the single experimental intensity. The ABR recording was achieved using 9 mm silver-silver chloride electrodes with 42" leads. Facial sites were prepared with Nuprep gel for vertex (Cz) and mastoid (M1, M2) electrode placement, with forehead (Fp) placement serving as ground. At least 1200 acceptable responses from the scalp were filtered using a bandpass parameter of 100-3k Hz, then amplified at 100k gain, and averaged. All stimulus rate conditions were repeated, providing at least two waveforms per rate, per ear for each subject.
Results
Data for analysis consisted of ABR wave V latencies derived at stimulus repetition rates of 31.1/sec, 45.1/sec and 61.1/sec. Means and standard deviations were calculated for the 20 subjects (40 ears) at each repetition rate and are as follows: 31.1/sec: 5.60 ms (.16); 45.1/sec: 5.73 ms (.17); 61.1/sec: 5.89 (.18). Figures 1a and 1b show Wave V latency shift with each stimulus rate change. Figure 1a shows average right and left ear latencies for each subject at each stimulus rate. Significant differences between right and left Wave V latencies were not found, suggesting the importance of averaging right and left ears together. Figure 1b shows Wave V latency shift with standard deviations. Statistical computations, including Pearson's r (.73 - .92), paired t test for the 31.1/sec vs. 61.1/sec rates (p<.0001 and variance covariance matrix tables for all mean comparisons were conducted.>
Discussion
The purpose of the study was to determine the degree of wave V latency shift with rapid stimulus repetition rate in normal subjects. This shift was found to be slightly and predictably greater for the 61.1/sec rate than for rates of 45.1 or 31.1/sec. Using the 61.1/sec rate diagnostically seems to make the test more sensitive to subtle auditory neurological lesions, including acoustic nerve tumors of 1.0 cm size and smaller. Therefore, a wave V latency of>6.25 msec (5.89 msec + 2 SDs) may be diagnostically sensitive to subtle neurological lesions when a 61/sec stimulus repetition rate is used.
Statistical analysis of the findings indicate that using two standard deviations from the mean latency of wave V while using a repetition rate of 61.1 clicks per second could detect a positive finding for a small acoustical tumor. Two standard deviations would be .36 or a 6.25 msec latency of wave V. Using three standard deviations, or a latency of 6.48 clicks per second, might yield fewer false-positive cases but would miss some small tumors. In summary, employing a 6.25 msec wave V latency cutoff as standard protocol for referral may identify more patients with small tumors than would a different procedure with different instrumentation, while keeping false-positive rates low.
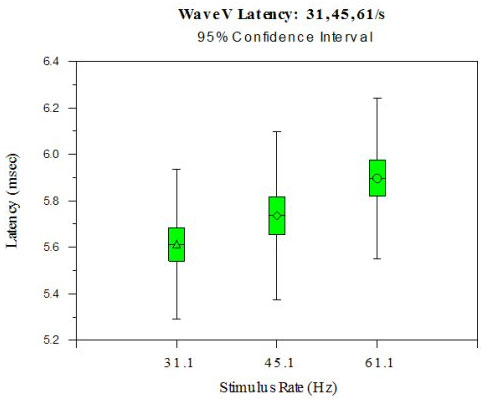
Figure 1a. ABR Wave V Latency is shown at three stimulus rates (31.1, 45.1, 61.1/s). Wave V latency shift is predictable (~0.10 ms) with an increased rate from 31.1/s to 45.1/s and from 45.1/s to 61.1/s.
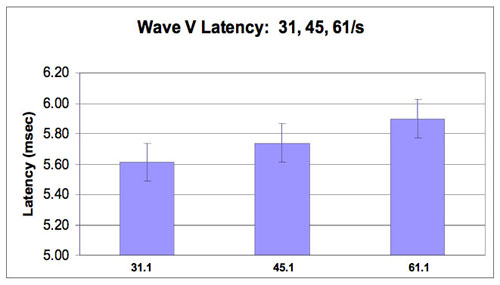
Figure 1b. Wave V Latency shift at 31.1, 45.1 and 61.1/s rates with standard deviations (0.16-0.18).
Case Study
The subject was a 46 y/o female presenting with sudden sensorineural hearing loss in her left ear. She denied tinnitus or vestibular symptoms.
Audiogram
A mild, high-frequency loss was identified in the right ear. The left ear demonstrated a trough-shaped, mild to moderately-severe sensorineural hearing loss (Figure 2).
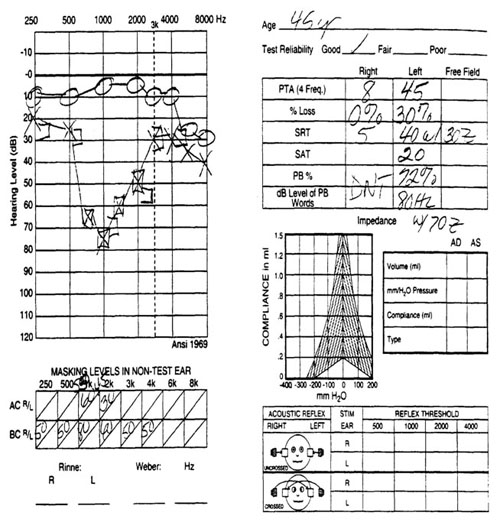
Figure 2. Case study audiogram.
ENG Findings
Positive electronystagmography (ENG) findings included spontaneous right beating nystagmus and a left reduced vestibular response of 44%.
ABR results
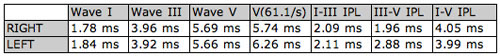
Wave V latencies and interpeak latencies (I-III, III-V, I-V) were normal bilaterally. Wave V was evident in the intensity series for the right ear, but was not well defined below 80 dB nHL for the left ear. The Wave V derived using rapid stimulus repetition rate (61.1/sec) was normal for the right ear, but shifted in latency in the left ear from 5.66 ms to 6.26 ms (>.4 ms) and had poor repeatability (Figure 3 and Table 2).
Interpretation
ENG results may be interpreted as a vestibular disorder of relatively recent onset, given marked spontaneous right beating nystagmus. The left ear is implicated with reduced caloric response, with the direction of spontaneous nystagmus beating opposite the affected side. In the context of the audiogram, history of sudden onset of hearing loss and basic ABR results, the possibility of vascular accident to the left ear could be considered. These ABR findings, if interpreted using conventional test protocol and diagnostic criteria, would likely give an erroneous interpretation of 'no retrocochlear pathology', which would be consistent with inner ear stroke. Indeed, poor wave repeatability in intensity series for the affected ear might be interpreted as severe cochlear damage, but would constitute yet another erroneous interpretation consistent with inner ear stroke. Although a significant wave V latency shift (>.4 ms) using a stimulus rate of 73.1/sec lends itself to retrocochlear pathology, which does not entirely rule out the possibility of vascular accident, it would, in this context, be secondary to tumor compression on the cochlear vein within the internal auditory canal which is a shared space with the VIIIth cranial nerve. The alternative diagnosis would be a tumor compressing the VIIIth cranial nerve, thus inducing hearing loss.
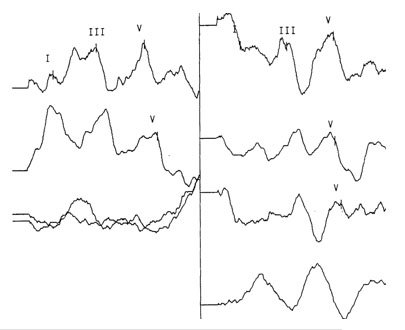
Figure 3. ABR Waveforms (Right and Left)
Table 1. Latency values for right and left ears
MRI Indications
High resolution fast spin echo T2 axial and coronal images of the internal auditory canals were obtained. Thin section T1 axial images were taken before and after injection of gadolinium with thin section coronal sections after contrast. A 'well-circumscribed 6 x 10 mm mass' was identified on the left side, which was predominantly within the internal auditory canal. The right internal auditory canal was normal.
Surgery
The patient opted for a hearing preservation surgical procedure to remove the tumor. A retrosigmoid approach was successfully performed. Post-operative results showed a recovery of hearing sensitivity on the left side to levels within the normal-hearing range.
This report describes a sudden, unilateral, sensorineural hearing loss in a 46 year-old female that could have been initially interpreted as a case of inner ear stroke. Test findings, including audiometric evaluation, ENG and ABR results, erroneously support this interpretation. However, a procedure used to sensitize the standard ABR protocol by 'over-adapting' the auditory nervous system with a rapid stimulus repetition rate produced a positive finding. MRI confirmed the intracanalicular location of the small tumor, and surgery successfully restored hearing sensitivity to the affected side. Based on these results, it is suggested that adding a rapid stimulus rate paradigm to the neurodiagnostic ABR workup might increase the identification of small acoustic neuromas.
Acknowledgements
Special thanks to Dr. Elizabeth Hellmuth, Ruth Marin and Dr. Danielle Inverso, Gallaudet University, for editorial comments and suggestions.
References
Barrs, D. M., Brackmann, D. E., Olson, J. E., & House, W. F. (1985). Changing concepts of acoustic tumor diagnosis. Archives of Otolaryngology, 111, 17-21.
Bauch, C.D., Olson, W.O. , Pool, A.F. (1996). ABR indices: sensitivity, specificity and tumor size. American Journal of Audiology, 5, 97-104.
Burkard, R. (1994). The use of maximum length sequences to obtain brainstem auditory evoked responses (BAERs) at rapid rates of stimulation. American Journal of Audiology, 3, 16-20.
Burkard, R., Hecox, K., (1983). The effect of broadband noise on the human brainstem auditory evoked response. I. Rate and intensity effects. Journal of the Acoustical Society of America, 74, 1204-1213.
Burkard, R. and Sims, D. (2004). The human auditory brainstem response to high click rates: Aging effects. American Journal of Audiology, 10, 1-9.
Coelho, A., & Prasher, D. (1990). Brainstem potentials in the diagnosis of an acoustic neuroma. Scandanavian Audiology, 19, 257-262.
Cheng, G., Smith, R. and Tan, A.K.W. (2003). Cost Comparison of Auditory Brainstem Response versus Magnetic Resonance Imaging Screening of Acoustic Neuroma. The Journal of Otolaryngology, 42, 394-399.
Daly, D. M., Roeser, R. J., Aung, M. H., & Daly, D. D. (1977). Early evoked potentials in patients with acoustic neuroma. Electroencephalograph and Clinical Neurophysiology, 43, 151-159.
Don, M., Allen, A., & Starr, A. (1977). Effect of click rate on the latency of auditory brainstem responses in humans. Anals of Otology, Rhinology and Laryngology, 86, 186-195.
Hood, L. J. (1998). Clinical applications of the Auditory Brainstem Response. Clifton, Park, Thompson Delmar Learning.
Jacobson, J., Murray, T. & Deppe, U. (1987). The effects of ABR stimulus repetition rate in multiple sclerosis. Ear and Hearing, 8, 115-120.
Jung, R, & Berger, W. (1979). Fiftieth anniversary of Hans Berger's publication of the electroencephalogram. Archives of Psychiatric Nervenk, 227, 279-300.
Lightfoot, G. R.(1992). ABR screening for acoustic neuromata: the role of rate-induced latency shift measurements. British Journal of Audiology, 26, 217-27.
Robinette, M. S., Bauch, C. D., Olsen, W. O & Cevette M. J. (2000). Auditory Brainstem Response and Magnetic Imaging for Acoustic Neuromas: Cost by Prevalence. Archives of Otolarygology Head Neck Surgery, 126, 963-966.
Robinson, K. & Rudge, P. (1977). Abnormalities of the auditory evoked-potentials in patients with multiple sclerosis. Brain, 100, 19-40.
Schmidt, RJ, Sataloff, RT, Newman, J, Spiegel, JR, & Myers, DL. (2001). The sensitivity of Auditory Brainstem Response Testing for the diagnosis of acoustic neuromas. Archives of Otolarygology Head Neck Surgery, 176, 19-22.
Selters, WA, & Brackman, DE. (1977). Acoustic tumor detection with brain stem electric response audiometry. Archives of Otolarygology, 103, 181-187.
Stockard, J. J., Sharbrough, F. W., & Stockard J. E. (1977). Detection and localization of occult lesions with brainstem auditory responses. Mayo Clinic Proceedings, 52, 761-769.
Tanaka, H., Komatsuzaki, A.S., & Hentona, H. (1996). Usefulness of auditory brainstem responses at high stimulus rates in the diagnosis of acoustic neuroma. ORL J Otorhinolaryngol Related Specialities, 58, 224-8.
Zappia, J. J., O'Connor, C. A., Wiet, R. J., & Dinces, E. A. (1997). Rethinking the use of auditory brainstem response in acoustic neuroma screening. Laryngoscope, 107, 1388-1392.

