Editor’s Note: This text course is an edited transcript of a live webinar. Download supplemental course materials.
Dr. Jennifer Wolf: Today’s course is the second training in the series Advanced Management of Complex Cases. Today we will focus on enlarged vestibular aqueduct (EVA).
Learning Objectives
After this course, you should be able to describe EVA and the theories of hearing loss, describe the audiologic test battery for differential diagnosis and possible audiologic configurations of hearing loss, and describe causes for progression of hearing loss and audiologic rehab options.
Agenda
To give you a brief outline of the presentation, we will discuss the definition of EVA, the audiologic configuration and progression of hearing loss, theories describing the cause of hearing loss, certain audiologic rehabilitation options, cochlear implant considerations, and we will end with a case study for a gentleman with bilateral EVA and cochlear implants.
Anatomy
I wanted to begin with a brief anatomy refresher as we begin talking about measurements used to define EVA. Think about the ear canal, tympanic membrane, middle ear ossicles, and the bony labyrinth of the inner ear. In the inner ear, you have posterior and superior semicircular canals that join to form the common crus, or what is sometimes labeled as the common canal. This space also contains the vestibule, which houses the utricle and saccule of the membranous labyrinth, and then there is the cochlea. The vestibular aqueduct juts out inferiorly from the vestibule.
The vestibular aqueduct runs from the vestibule to the posterior cranial fossa and forms a J-shape. Running through each vestibular aqueduct is a fluid-filled tube called the endolymphatic duct, which connects the inner ear to a balloon-shaped structure known as the endolymphatic sac. Covering the endolymphatic sac is a cap of bone known as the bony operculum.
Mondini was the first to describe EVA as well as a bulbous endolymphatic sac in 1791 during a temporal bone dissection. He went on to identify additional abnormalities of the inner ear, including one and half turns of the cochlea rather than the usual two and a half. He saw semicircular canals that were small or missing and a dilated vestibule.
It was not until 1969 when Valvassori then reported Meniere’s-like symptoms in the presence of EVA. Valvassori and Clemis are then credited with defining EVA in 1978 and recognizing the relationship between hearing loss and vestibule aqueduct enlargement. They did this after retrospectively reviewing 3,700 tomographic studies and found that 50 patients out of the 3,700 had enlargement of the vestibular aqueduct, with the majority of those 50 having congenital hearing loss and many of them reporting vestibular symptoms. Of those 50 patients, 60% had additional structural abnormalities which included an enlarged vestibule, enlarged semicircular canals, hypoplastic cochlea or a combination of them.
Valvassori and Clemis then presented a definition in 1978, stating that EVA should be classified by a “diameter greater than 1.5 mm halfway between the common crus and medial aspect of the operculum on the posterior wall of the temporal bone.” Recall that the operculum covers the endolymphatic sac. In adults, the upper range of normal for a vestibular aqueduct is 0.9 mm at the midpoint and 1.9 mm at the operculum. To give you a point of reference, if you were to take three crystals of table salt and line then up next to each other, that would be equivalent to 1 mm. For patients with EVA, the midpoint will range from about 1.5 mm to 8 mm with the average in adult patients around 4 mm. A reference point for that diameter of 1.5 mm or greater is the head of a straight pin.
If you start looking at articles published on EVA, some authors use different criteria from the Valvassori and Clemis (1978) criteria. However, the one set forth by Valvassori and Clemis is still the most commonly used and accepted today. One additional size consideration that a radiologist will use is comparison of the size of the vestibular aqueduct to the size of the lateral semicircular canal.
Diagnosing EVA
Now that we have discussed the measurements defining EVA, how is it definitively diagnosed? The best way to do this is by imaging. Diagnosis can be made by a radiologist with either CT or MRI scans. There is a difference between the two. On a CT scan, the vestibular aqueduct can be identified, whereas on an MRI scan, the endolymphatic duct as well as the endolymphatic sac can be identified. The areas of measurement are still the same, but they structurally identify different anatomy.
Since imaging is now recommended in workups for etiology of hearing loss, especially for children, we have come to learn that EVA is the most common inner ear anomaly identified on imaging for children with hearing loss. While it is the most common, EVA will also concomitantly occur with other cochleovestibular anomalies roughly 40% of the time. This number is slightly different than the initial data by Valvasori and Clemis (1978), which reported that approximately 60% had other anatomical abnormalities.
Development
Unlike the remainder of the inner ear which is thought to be fully developed prior to birth, there is much controversy surrounding the growth of the vestibular aqueduct and when it is mature in size. Some believe that the vestibular aqueduct is complete in size and mature by birth, complete in utero. Others believe that there is continued growth post-natally.
Going back to the thought that the vestibular aqueduct is complete in utero, there is evidence that there is no change in the vestibular aqueduct size following birth, which is supported by imaging data suggesting that the diameter and the means of the diameter of the vestibular aqueduct, when compared between children and adults, did not significantly change.
Some also think that the vestibular aqueduct is initially wider and throughout fetal development, it elongates and takes on the J shape. However, in cases of EVA, there is some thought that the vestibular aqueduct remains wide. It does not elongate; this arrest in development is said to occur about five weeks’ gestation.
Contrary to this is the thought that the vestibular aqueduct continues to grow in to childhood. This is based off of a few different articles, with the most recent one published by Dr. Mark Pyle in 2000. He reported that the widest aqueduct for embryos during different stages of development was 0.32 mm at the midpoint and 0.88 mm at the internal and external apertures of the vestibular aqueduct itself. Based on his research, he has seen a progressive, nonlinear growth of the vestibular aqueduct throughout gestation, and he notes that the growth does not decline or reach maximum size during fetal life. His measurements are still smaller than the average reported.
He goes on to note that his work is supported by previous data, which suggests that the vestibular aqueduct continues to grow post-natally until the age of three or four. His research does confirm that the remainder of the membranous labyrinth is completely formed by 20 weeks’ gestation, with the exception that the vestibular aqueduct continues to grow throughout fetal life, and potentially postnatally until the age of three or four.
The physicians I work with do not disagree that there is a chance for continued growth. However, they deny seeing that someone who has a normal vestibular aqueduct would then end up with an enlarged vestibular aqueduct between birth and two years of life. They still believe that it is proportionally bigger at birth and may continue to grow a little bit.
Statistics
I want to review a few statistics. Know that as we come to learn more about the diagnosis of EVA, these will likely change over time. From all the data gathered thus far, the prevalence of EVA is estimated to range from 1% to 14% of populations with sensorineural hearing loss. The bilateral to unilateral ratio is 2:1, and the female to male ratio is 3:2.
In addition to occurring in isolation, EVA has also been shown to occur in conjunction with certain congenital disorders, such as Pendred syndrome, which is autosomal recessive. There are two mutations on the gene SLC26A4, which results in hypothyroidism and goiter. The combination of thyroid dysfunction and EVA sums up Pendred syndrome. Other syndromes that you may see in conjunction with EVA would be CHARGE syndrome or branchiootorenal (BOR) syndrome.
Clinical Presentation
Let’s discuss the signs and symptoms with which an individual may present at your clinic. First, there will likely be concerns of hearing loss, which is why they are coming to see you. They may have failed a hearing screening, whether that is the newborn hearing screening or a pediatrician/school screening. There may be reports of reduced auditory responsive in daily activities. In this case, a parent would notice that their child is no longer responding to their name being called from a distance, or when they are in a noisy restaurant, their child is having a harder time hearing.
This may be the case for young adults where spouses or friends are noticing it as well. This may be noticed following minor head trauma, or there may be no specific incident at all. The patients themselves may report difficulty hearing depending upon their age, or there may be some speech and language delay concerns or other diagnosis of something like apraxia. That may all bring a child or an adult to your door.
The vestibular symptoms may be that a child is delayed in crawling or walking, or there may be general concerns with imbalance or disequilibrium. A patient who can report their symptoms may describe episodes of vertigo that vary in length or a general sense of disequilibrium.
In our clinic at Cornell, we have seen that the majority of children presenting with EVA alone come in from pediatrician and/or school screenings around the ages of three to six, whereas children with other structural abnormalities of the inner ear may make their way in earlier due to referral on newborn hearing screenings. It is important to have a good referral relationship with pediatricians in the area and to encourage them not to undermine the importance of referrals on in-office screenings.
Audiologic Test Battery
Once the patient presents at your clinic, what type of tests do you want to use? For many of us, this is our typical test battery, including tympanometry, acoustic reflexes, pure-tone thresholds, word recognition, otoacoustic emissions (OAE) and vestibular-evoked myogenic potentials (VEMP). It is not common practice in some clinics to test bone conduction for 250 Hz or to test below 0 dB HL, but I urge you to reconsider these practices if you fall into this category. You will see evidence today as to why this becomes very important and may help build a case for diagnosis and further questioning of hearing loss and the etiology of hearing loss.
Type and Configuration of Hearing Loss
Conductive, mixed, and sensorineural losses have all been reported in the literature. In the case of EVA, conductive hearing loss or mixed components are most likely to occur in the low frequencies, such as 250 Hz, 500 Hz and sometimes up to 1000 Hz. Oftentimes, bone conduction can be very good and present at the lowest end of normal, such as -10 dB, which may be missed if you are not testing below 0 dB HL. It is likely to see those conductive or mixed components in the lows, but we often see sensorineural components in the highs. As hearing declines, whether it be in a stepwise fashion, a gradual change, or more of a sudden change in hearing, we tend to see the hearing loss shift from conductive or mixed to sensorineural.
The most common reported configurations of hearing loss for patients with EVA are downsloping, flat, and reverse cookie bite where the low frequencies have a hearing loss and hearing comes up and is better in the mid frequencies, and then becomes poorer again in the high frequencies.
Degree of hearing loss can be all over the audiogram. There is no rule here. Recall that many patients with EVA will have additional inner ear abnormalities that may impact the progression or the degree of hearing loss. The degree of hearing loss can range from mild to profound. It is reported to potentially fluctuate, rapidly change, or gradually change over time with no specific incident or head injury. Hearing loss can also range from deafness in childhood to stable hearing loss into adult life.
A review completed in 2011 by Dr. Gopen and colleagues revealed that 30% to 40% of ears had a stable hearing loss from a seven to nine year period, meaning that roughly over nine years, about 30-40% of ears had stable hearing. While that is great, we have to consider the other 60% of patients who have a gradual progression or sudden changes in hearing.
Hand in hand with pure-tone thresholds, we likely will test word recognition ability. Oftentimes, word recognition will decline with the progression of hearing loss, just as we would see with typical progressive hearing losses, although word recognition may be poorer than expected when compared to other individuals who have conductive or mixed components of middle ear origin. Often in those cases, as soon as we bring the intensity up to a louder level, patients do very well on word recognition. This is not always the case in EVA, despite the presence of the mixed or conductive components.
Additional measurements we include in our clinical workup will become very important. If we have a patient in front of us with a conductive and mixed component, our goal with tympanometry is to determine if the eardrum is moving well or if there is negative pressure in the ear. Even in the presence of a conductive or mixed component for patients with EVA, tympanometry is expected to be within normal limits, given that there is no fluid there.
Acoustic reflexes performed with either tonal stimuli or broadband noise can typically be present with conductive or mixed components. If there is a straight sensorineural hearing loss in that ear, the general rules apply where we would expect the reflex to present, elevated, or absent. If you have a normal tymp, a conductive or mixed component and you are obtaining reflexes, that is an indication that the ossicles are moving as they should; that pattern is helpful in ruling out middle ear pathology. In EVA we are not looking to the middle ear as the source of the issue, so oftentimes reflexes are present.
OAEs can be present even in the presence of air-bone gaps. However, it is typically consistent with the sensorineural patterns. If someone has more than a mild to moderate hearing loss, OAEs are generally absent. If you have someone with what appears to be a slight conductive hearing loss and you are able to record OAEs, that also likely rules out middle ear pathology.
One other test that may not be part of a typical battery is VEMP testing. VEMP will often be present despite air-bone gaps. In true middle ear pathology, we are not able to obtain a VEMP response. In cases with EVA, there is the potential for high ocular VEMP amplitudes and low cervical VEMP thresholds. Even though there is conductive or mixed components, we are expecting to record a VEMP. C-VEMPs are most common clinically, and we are expecting to record them down to a low threshold level in some cases. On the lower end, they can be along the lines of 65 dB.
We want to question EVA in cases where we have someone with conductive or mixed hearing loss who presents with Type A tympanograms and present reflexes. We also want to consider this when language for children is superior than is expected given the hearing loss they are presenting with, which could indicate a progression over time or recent head trauma followed by a reported decline in hearing. These conditions raise red flags. In the presence of a conductive or mixed component, it is helpful to complete the tests as seen in Figure 1, as responses will often be absent if there is true middle ear pathology.
Figure 1. Testing results used for differential diagnosis of EVA.
However, based on the testing here, it may still be difficult to differentiate between other third window pathologies such as semicircular canal dehiscence or to rule out something like a perilymphatic fistula. This is extremely helpful as we pass these results on to a physician. It may deter them from something like middle ear exploration to figure out why there is a conductive or mixed component there.
Describing Conductive or Mixed Loss with no Middle Ear Pathology
Given all of these results, how do we adequately describe the presence of a conductive or mixed component when they are not consistent with middle ear pathology?
What nomenclature is best? In addition to the traditional descriptions like mixed hearing loss, sensorineural, or air-bone gap, terms such as pseudoconductive or cochlear conductive have been used, but it is recommended to stay away from those because they tend to not be very clear to some physicians. It may be best to note that there are air-bone gaps in the presence of immittance findings consistent with normal middle ear function.
As audiologists, not technicians, we want to interpret these results. This is part of our job. Yes, there is a mixed or conductive component. The middle ear system is working normally from what we can tell, but there is still a hearing loss. When we are interpreting the results, we want to describe the conductive components and air-bone gaps, and we want to indicate that despite the conductive components, it is not consistent with middle ear pathology.
Precipitating Factors
There is a good chance that hearing will fluctuate or suddenly decline, because only 30-40% of patients had a stable hearing loss over a period of up to nine years. Is there a way to counsel patients on the potential causes of change? Are there specific factors that can increase a patient’s risk for these sudden changes? The answer to this is yes.
Head trauma, barotrauma, high fever, noise exposure, and upper respiratory infections can all increase the risk for sudden changes in hearing. Some of these can be more easily avoided than others. We want to make sure that patients know that these could be factors and causes of a further decline in hearing. However, despite previous belief, minor head trauma does not always cause a further decline in hearing. Only one third of patients reported a sudden decline in hearing following minor head trauma.
For example, say a child with EVA sliding down a slide accidentally bumps into his friend at the end, hits his head, and falls down. It was thought at one point that regardless of the head injury or trauma, there would be an immediate change in hearing. That is not necessarily the case.
Norman et al. completed a meta-analysis in 2014 that also indicated that the odds for experiencing a sudden hearing loss following minor head trauma was 8.6 times higher in patients with pre-existing fluctuations in hearing. If you have a patient whose hearing tends to fluctuate all over the audiogram, you may advise them against any sort of potential head trauma or barotrauma.
In the majority of studies, the vestibular aqueduct size and endolymphatic sac size is not correlated with the degree of hearing loss. There is no way to predict if someone is more likely to experience a sudden or rapid decline versus a gradual decline of hearing over time. However, Madden et al. (2003) reported that the mean vestibular aqueduct at the operculum was significantly larger in patients with progressive hearing losses versus those with stable or fluctuating losses.
Clinical Implications
If we cannot predict the potential changes but know what some of them are, what is the best way to manage these patients? Monitor, Monitor, Monitor! It is important to closely monitor hearing given the potential changes that patients may incur, especially for children; they may not be able to express their concerns at a young age.
We want to use all of the diagnostic tools possible in order to help a physician make a correct diagnosis and avoid unnecessary surgery. We want to counsel patients and families on potential causes of further hearing losses and activities they may wish to avoid, such as contact sports like football or soccer, and potentially wind instruments, where the intracranial pressure increases.
Additionally, there have been reports of rapid changes in hearing and spontaneous recoveries. This should be considered with hearing aid programming, especially if it is a child who may not be able to provide clear feedback.
Case Study A
The following cases are from patients we have seen in our clinic.
The first patient presented to our clinic for a second opinion due to reported inconsistent results, particularly regarding her bone conduction thresholds. Her parents brought with them previous evaluations, detailing fluctuations in low-frequency bone conduction, sometimes considered conductive on tests, but more recently was mixed. In all of these cases, tympanograms and reflexes were normal and present.
The patient was diagnosed with congenital stapes fixation. The physician recommended waiting until the child was older to complete a CT scan. I she was four or five years old at that time. On the day that she was seen at our clinic, she had normal tympanograms, present reflexes, and the thresholds seen in Figure 2.
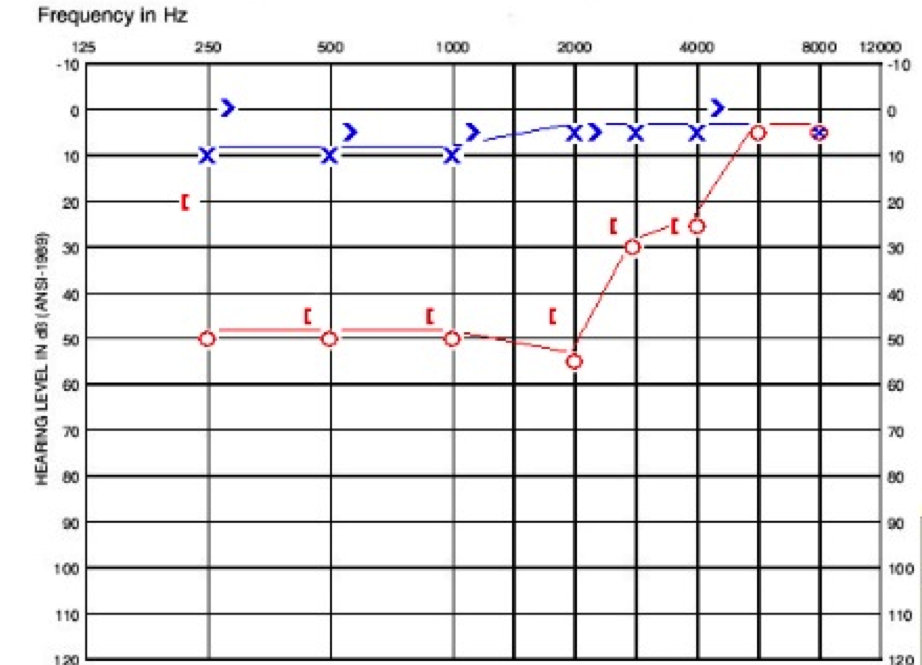
Figure 2. Audiogram of child in case A.
Her left ear is within the normal range. Her right ear has a moderate conductive hearing loss at 250 Hz, followed by a moderate rising to slight sensorineural hearing loss. Her word recognition was excellent in both ears with 96% on the right and 100% on the left. She had a CT scan at our facility and was diagnosed with right EVA. She was fit with a hearing aid, and her hearing loss appears stable.
Case Study B
The next patient is seven years old. She presented at our clinic after referring on a screening at her pediatrician’s office. Her parents noted that she was being evaluated for reading difficulties and possible dyslexia at school. She was born premature and had two-week NICU stay, although no ototoxic treatment was reported. She had no history of otitis media or family history of hearing loss.
Her tympanograms were normal on the day she presented to us. In the right ear, she had acoustic reflexes present at 500 Hz, 1000 Hz, 2000 Hz, and 4000 Hz, whereas on the left, acoustic reflexes were present at 500 Hz and 1000 Hz only. Her word recognition was 100% in the right ear and 96% in the left. These findings are shown in Figure 3.
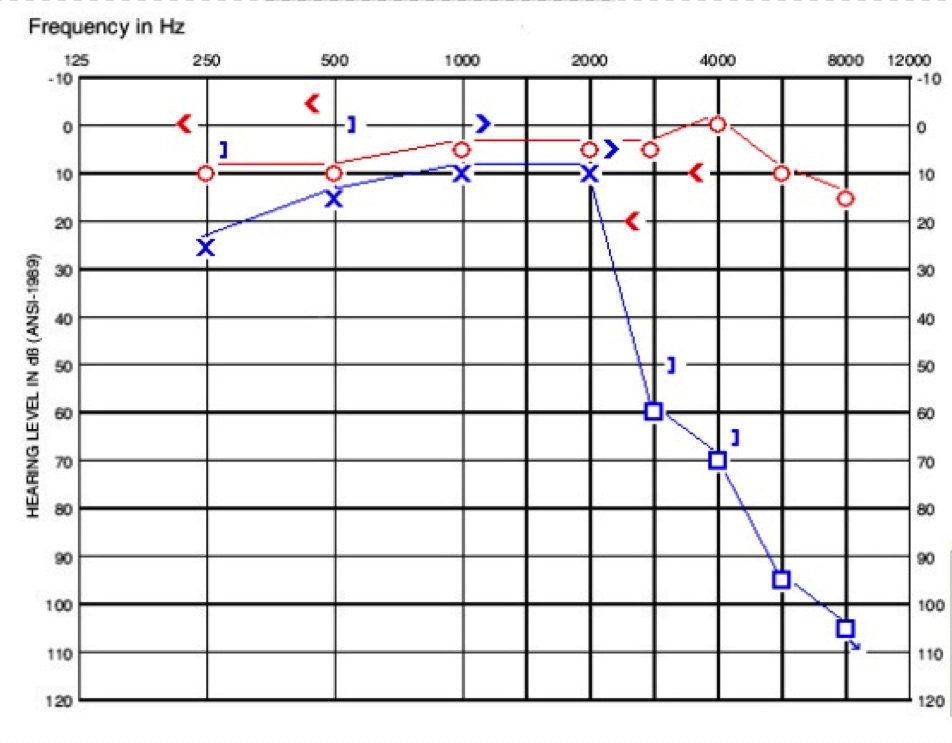
Figure 3. Audiogram of child in case B.
She then underwent a CT scan which revealed a large vestibular aqueduct in the left ear measuring over 3 mm at the midpoint. Otherwise, her left cochlea and labyrinth were normal. Her hearing has luckily remained stable over a two-year period. She uses a receiver-in-the-canal (RIC) hearing aid and a ConnectLine mic at home. She uses an FM system in school with success.
The take-home point from this particular case is that language or reading concerns may be underlying hearing losses. Despite the thought that one ear is adequate for speech and language development, many children need additional accommodations to keep on par with their normal-hearing peers.
Case Study C
This next patient presented to our clinic with a diagnosis of EVA already. He has an interesting history because he had referred on his newborn hearing screening. He had a moderate conductive loss on his auditory brainstem response (ABR) test, and he had abnormal tympanograms and absent reflexes at that time, according to another clinic’s report. He had pressure equalization tubes placed due to consistent hearing loss and abnormal tymps. He was monitored, but unfortunately his hearing never recovered to where we would hope it would be following tube placement.
He presented at our clinic for a change of care. The day we saw him, we did not test reflexes or OAEs as he still had tubes placed. His right ear had more of a steeply sloping sensorineural hearing loss, and the left ear had a flat sensorineural hearing loss with a mixed component at 1000 Hz (Figure 4). We would have liked to get more information from him, but he fatigued to the testing.
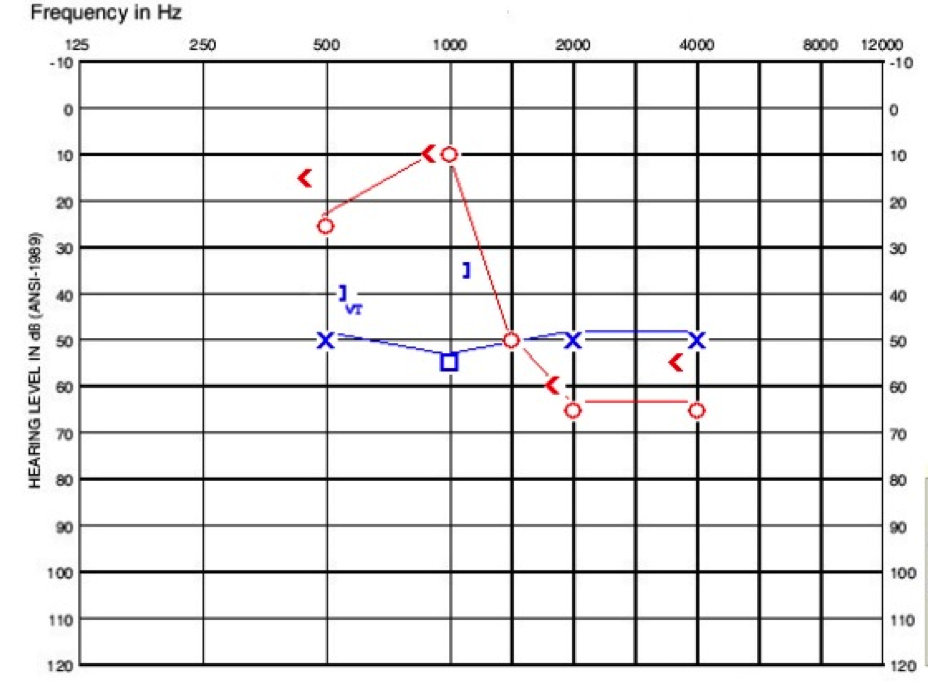
Figure 4. Audiogram of child in case C.
The take home point for this patient is that EVA may be masked by other conductive losses thought to be of middle ear origin and fluid related, but it is important to keep questioning remaining losses following tube placement where hearing is not resolving to normal.
Case Study D
The next patient is a six-year-old who referred on his initial newborn hearing screening, but passed the repeat evaluation. He had a one-day NICU stay for monitoring. He was receiving speech services at the time he presented to us due to reported difficulty articulating fricatives. That was a red flag to us. He also had a working diagnosis of apraxia.
He referred on his pediatrician screening at the age of three. There was no family history of hearing loss or ear infections. When we saw him, he had normal tympanograms. He had present reflexes on the right and absent reflexes on the left. His audiogram is shown in Figure 5, where you can see a low-frequency conductive loss on the right side and then it changes to more of a sensorineural loss. The left ear is poorer, sloping from moderate to profound sensorineural loss.
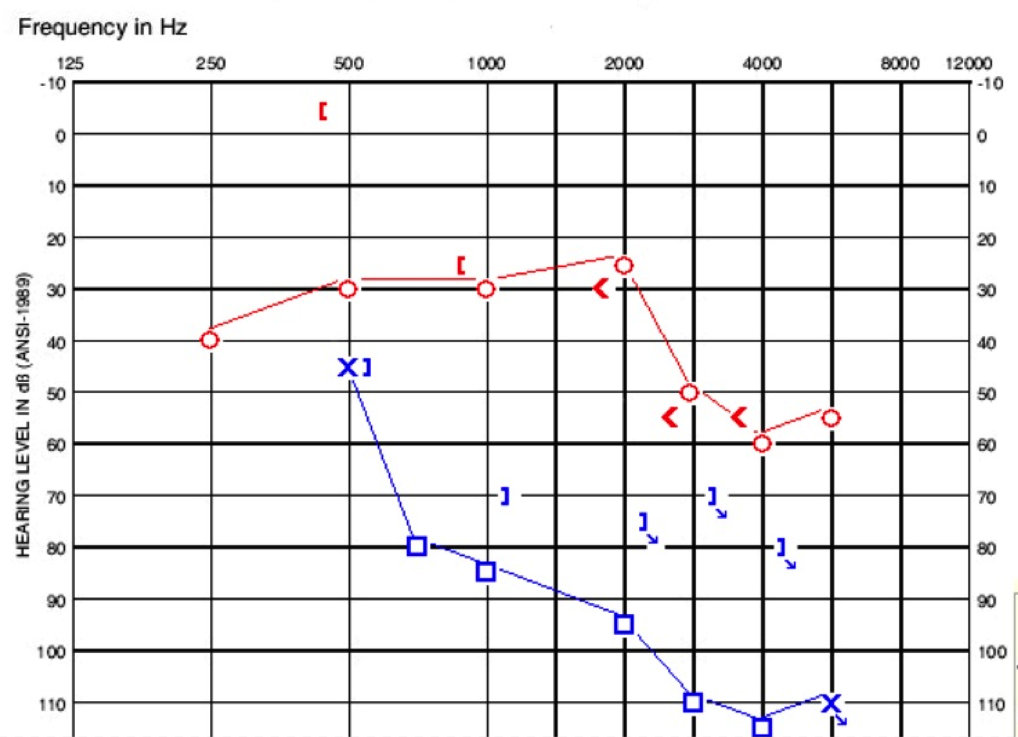
Figure 5. Audiogram of child in case D.
When we saw him, my colleague completed the WIPI (Word Intelligibility by Picture Identification) due to articulation concerns, and there was a significant difference in word recognition between the two ears. He was fit with binaural amplification and is a great child who is able to articulate how he hears very well.
When he underwent his CT scan, it revealed bilateral EVA with vestibular aqueducts up to 8 mm at the midpoint. He also had incomplete partition type II and reported deficiencies of the modiolus bilaterally. Unfortunately, he has had a progression in hearing loss, which is shown in Figure 6. He is now being considered for a cochlear implant on the left side due to continuing rapid changes in word recognition.
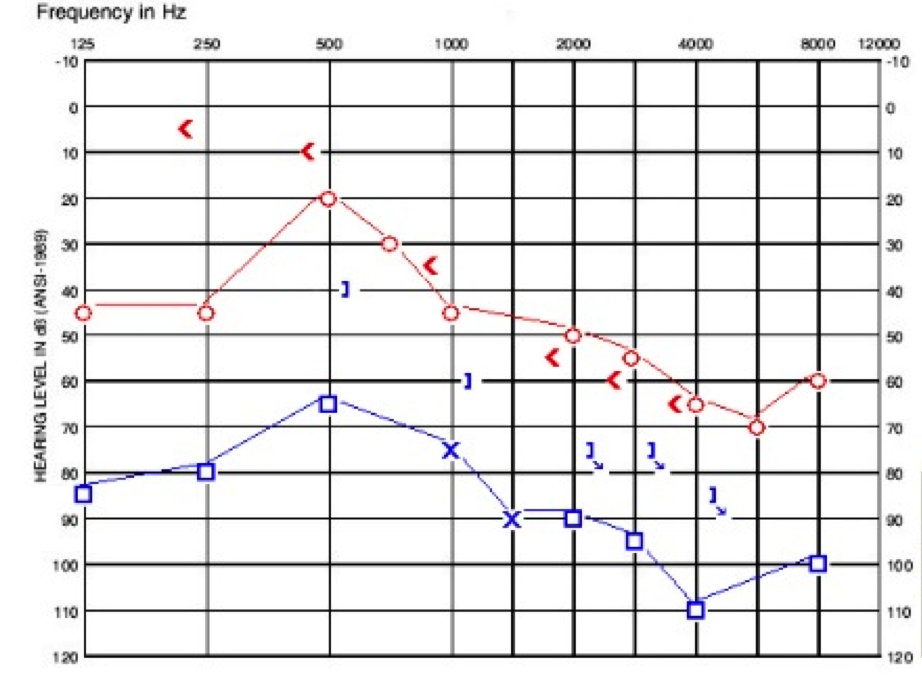
Figure 6. Case study D audiogram, one year after presenting in clinic. Compare to Figure 5.
While it is tempting to focus solely on pure tones, when word recognition is on the decline, we may want to begin discussing moving from hearing aids to cochlear implants.
Case Study E
This is a patient who presented at our clinic reporting hearing loss diagnosed in childhood. She said that her left ear was always her better-hearing ear until taking a flight abroad, after which she noticed a sudden decline in hearing and the onset of subjectively loud tinnitus. She was treated with oral steroids abroad. However, her hearing did not recover.
When she returned to America, she underwent a CT scan and was first diagnosed with EVA. She decided not to move forward with a cochlear implant as she wanted to hold on to any hearing that she had through her hearing aids. She felt that she received environmental sound awareness, which was important to her, and she was not willing to potentially give that up for a period of time to be implanted. Hopefully we will see her come back to the clinic in the future.
Theories of Hearing Loss
Some believe that EVA is a radiologic marker for hearing loss and not the cause itself, whereas other suggest that EVA is the cause of hearing loss.
Pressure Waves
There are two pressure wave theories, with the first being that conductive or mixed losses can be explained by the back pressure of perilymph and endolymph, causing decreased stapes mobility. They also state this as a potential reason for perilymphatic gushers during surgery of the middle and inner ear. However, this does not explain the sensorineural components we often encounter.
The second pressure wave theory also says that the greater pressure shifts from the intracranial space cross through the vestibular aqueduct and damage the inner ear, specifically the hair cells.
Electrolyte Imbalance
Other theories include the electrolyte imbalance theory, which proposes that the endolymphatic sac itself disturbs homeostasis of the inner ear, and large volumes of endolymph could overwhelm the ion pump mechanism of the stria vascularis. Along the same lines, some suspect that endolymphatic duct fluid can be toxic if in large quantities, and can contain hyperosmolar fluid, which can reflux through the ear or the larger aqueduct and cause damage to the inner ear, based on the quantity and makeup of the fluid.
Middle Ear Contributions
Some point to the middle ear as having some involvement for mixed or conductive components, indicating that in certain situations in patients, there have been noted ossicular deformities or stapes fixation, which has been found on middle ear exploration prior to imaging.
Third Window Lesion
One other theory is the third window lesion, or any abnormal entrance to the inner ear; this does not refer to the oval window or the round window. This is suspecting that the abnormal opening changes the compliance of the system and results from sound being shunted out of the cochlea. This decreased compliance allows for the enhancement of bone conduction. Therefore, mixed components mean very good bone conduction in some cases and poorer air-conduction thresholds. Again, sound energy is shunted out causing poorer air conduction and improved bone conduction.
A further description can be found in the article by Merchant and Rosowski (2008). Merchant and colleagues also published a paper in 2007 theorizing that the air-bone gaps would be largest below 2000 Hz due to anatomic dimensions of the ear and physics of sound. They also state that the underlying sensorineural hearing loss would potentially mask out these air-bone gaps as the disorder continues to progress.
Treatment for Declining Hearing
Attempted treatment for EVA has included endolymphatic sac shunting, occlusion, or obliteration surgery. However, none of these have been proven effective in treating the hearing loss, and in some cases, makes it worse or a patient will have a sudden change immediately following the procedure with no recovery.
Corticosteroid treatment has also been used, stemming along the lines of when patients come in with sudden sensorineural changes in hearing. Oftentimes steroids, whether oral or intratympanic, are completed or provided to see if there is a recovery in hearing. Nothing has been proven effective with this in patients with EVA.
Since there are no proven effective treatment options, we want to consider all the potential ways we can aid these patients in communication, which will include preferential seating for a child in the classroom, FM systems (sound field or personal), hearing aids, cochlear implants or a combination of the above, depending on the degree of hearing loss and word recognition ability.
In our clinic, we allow the patients to steer these decisions and tell them that they can move forward with a hearing aid, even if it is someone with a mild hearing loss, to see what they think and how they feel about their hearing day to day. In the majority of cases, it is often helpful to give them all of their options and then allow the patient to decide. As we move through our options, if hearing aids are not enough, then we talk about cochlear implants.
Cochlear Implant Considerations
First and foremost, a mixed component should not rule out cochlear implant candidacy. If you look through the literature on EVA and progression of hearing loss, many cases exist where patients have not moved forward because there was still a mixed or conductive component. We do not know the exact reason as to why patients are presenting with this mixed component, and in the majority of cases, there is no middle ear involvement. That should not be holding them back from being potential candidates. Physicians should be focusing on imaging to rule out other medical involvement, and we should rely greatly on word recognition ability.
Surgical Considerations
One surgical aspect to consider with EVA is the electrode array. The array may be dependent on other anatomical considerations, knowing that for a good number of these patients, they will have other abnormalities of the inner ear and also progression of hearing loss. Even though someone may have a sloping hearing loss, do we want to preserve that hearing by using a hybrid device when there is a good chance that their hearing loss will progress?
Additionally, there are reported gushers in the literature. This is when a surgeon drills a cochleostomy and perilymph is leaking out. That is something that physicians will consider in a surgery and well as post-operative recovery. Some of these patients may have increased dizziness following the procedure, as they had vestibular symptoms to begin with.
Implant Programming
When it comes to cochlear implant programming, I have seen some patients that have the potential for fluctuations in impedances, which can affect their voltage compliance limitations. It is very important to keep an eye on impedances and document if there are fluctuations. There could be different preferred loudness measurements. Make sure that you are completing loudness growth throughout your programming sessions. In bilateral patients, you may want to allow them complete control of each ear independently, so if there are fluctuations, they can make changes as needed. Obviously, this is case-by-case with precautions noted for children. Something like QuickSync may not be the best option for someone with bilateral EVA.
Performance
As far as performance, many children will have acquired some speech and language before they are implanted, and may have limited time without auditory input. In those cases, children tend to do well, even if they had profound hearing loss from birth. We know if they are implanted early with the right support and services, they tend to do very well.
Many adults with EVA, however, tend to hold on tightly to even a small amount of hearing. They tend to be fearful of implanting their ears because they have lived their whole life with gradual or sudden progressions of hearing loss, and they use up all of that auditory information they may have with their hearing aids. In addition, the duration of hearing loss in previous use of amplification can impact post-operative performance.
Case Studies
Cochlear Implantation with Bilateral EVA
This patient is a 40-year-old male with bilateral, progressive hearing loss that was first identified when he was four. He noted poor hearing always in the left ear from childhood, he consistently used amplification in the right ear, and he was able to access speech. In the left ear, he reports more environmental sound awareness and vibrotactile sensations later on with the progression of hearing loss.
He came into our clinic and met with the surgeon first, who immediately referred him for a CT scan. Before he saw me for his implant evaluation, he was diagnosed with EVA at the age of 38. For him, it was a sense of relief of knowing why his hearing had been declining. To him, it made sense.
Figure 7 shows his pure-tone thresholds. At that time I could not plot at 125 Hz on our audiogram, but it was 90 dB on left and 95 dB on the right. By pure-tone standards, he was a good cochlear implant candidate.
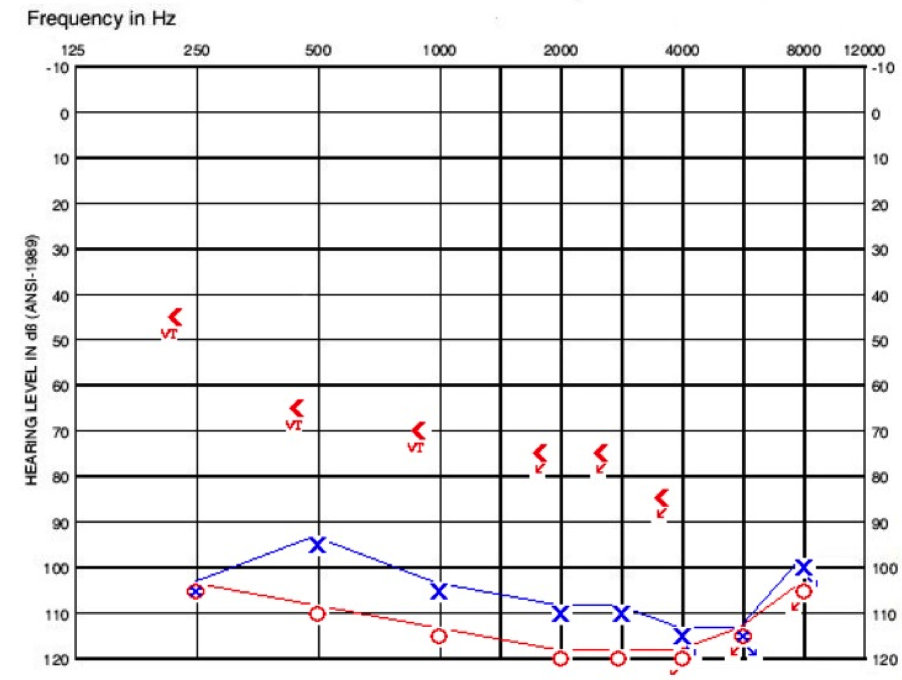
Figure 7. Cochlear implant evaluation audiogram for patient with bilateral EVA.
His aided evaluation showed 0% on the AzBio, with minimal information on the CNC (consonant-vowel nucleus-consonant) and HINT (Hearing in Noise Test). I chose to do a four-choice spondee test with him. We tend to do this and the HINT in cases where patients are not able to repeat back any information on the AzBio or CNCs as a starting point for post-operative performance in comparison.
He ended up moving forward with a cochlear implant and chose Advanced Bionics. He was implanted in the right ear first with a HiFocus 1j electrode. There was no resistance on insertion, and no evidence of a gusher per physician report.
I saw him for activation. He was fitted with a Naída Q70 processor, and we chose the HiRES OptimaS processing strategy. This patient’s activation is what we all dream of. His son was sitting underneath the table, and his wife, mother, and sister were all in the room. His impedances looked good. His NRI (neural response imaging) measured from the operating room was ideal.
His tolerance levels were great. We measured M levels and then dropped them down. I do loudness growth across the ear with these patients. Once I turned on the mics and started bringing up the input, he heard his son under the table who was playing with his toys. During activation, he had closed-set speech understanding. I work with patients to do the /s/, and we work through spondees and monosyllabic words in the closed-set task. He was doing unbelievably well and was even able to answer basic questions without visual cues on the day of his activation.
His performance with a cochlear implant is what we all hope for. Figure 8 shows his speech performance at the three-month and one-year mark.
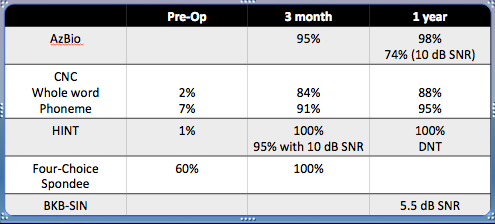
Figure 8. Post-operative speech performance over a year.
At the one-year mark, he decided that he may want to move forward with the second side. Given his history of only environmental sound awareness and vibrotactile sensations with amplification, we talked a lot about what that may mean when it comes to a cochlear implant and the variability of performance outcomes. He decided to move forward with his left ear and was implanted with a 1j electrode array on the left side.
Activation for that side was a little different. He did have NRI, although the numbers were much higher for this ear than his right ear had been in the operating room. At the day of activation, he was reporting that he could hear environmental sounds coming through the processor, but he was not able to understand speech. Unfortunately, that still remains the case today. However, he finds significant benefit from that second side when it comes to localization.
He says it helps him with speech reading when he does need to utilize those cues, and he can still hear the intonation of speech, but cannot understand speech clearly, compared to his better ear.
Summary
I thought his case was interesting considering we had an ear that gradually declined and was more recently deafened. Previously, he received benefit from amplification versus an ear that had a longstanding hearing loss and with limited benefit from amplification. He is an ear-to-ear comparison within one subject.
The counseling aspect is very important, especially with cochlear implants. We hope that he will progress with his implants and continue to find benefit. We played around with the processing strategies, and I say this knowing that if someone were to open up his programming report, they would cringe and ask why he is programmed with OptimaS on one side and OptimaP on the other. This was due to his reported sound quality that was better when we moved to a paired strategy on the poorer side. That is what he likes and he controls each ear independently with on-ear controls.
Recently, I received an e-mail from his wife, who sent me the audiogram of their son saying he has been monitored of his hearing. On that hearing test, he unfortunately was diagnosed with hearing loss in one ear and subsequently had a CT scan done, which showed a right EVA. This condition can be genetic, so be sure that one of your recommendations is to test other family members as well.
Conclusion
In summary, hearing loss can present as conductive, mixed, or sensorineural in patients with EVA. It can remain stable, fluctuate, or rapidly change. When EVA is suspected, we should use all of the clinical tools we have to help decipher a conductive or mixed component, middle ear origin from inner ear origin, and we should review the potential precipitating hearing loss factors with patients and families once they are diagnosed with EVA.
Most importantly, we should monitor hearing, hearing aid performance, and cochlear implant programming closely for potential fluctuations. If you are working with patients who have EVA and are interested in being involved in research, NIH is currently conducting studies and recruiting participants with EVA (https://clinicalstudies.info.nih.gov/detail/A_2001-DC-0228.html).
Questions and Answers
When programming hearing aids for patients that present with a conductive component, should the bone thresholds be used for setting and verification of the hearing aid, or should the loss be considered sensorineural for this purpose?
That is a good question. I do not think there is a hard-and-fast rule for that. Considering all the information that we have that EVA is likely not originating from the middle ear, you may want to program it for the sensorineural loss. You would want feedback from the patient, if possible. You can try programming it both ways to see if performance is better in one condition over the other. I would definitely verify the fitting electroacoustically as well as in the booth, including word recognition. The other thing you can do is have parents (or teachers) keep documentation of their child’s behavior and responses if you do change the settings.
Following implantation, how frequently do you see continued fluctuations in hearing?
Oftentimes, these patients they are being implanted with a full insertion, and we are not trying to preserve any low-frequency components. We are not monitoring pure tones once they are implanted, unless they have some unordinary sound percept or if there are other concerns going on. As far as speech perception with a cochlear implant, I tend to put my patients in the booth more than they want. Every time they come in for a programming session, I tend to put them in the booth. Within the first three to six months, I am test them three to four times, usually starting off with just detection in the sound field, moving into word recognition ability. Once they are implanted for about six months, I see them every three to six months. If there are even small fluctuations in hearing or impedances or their sound percept, I will see them every three to six months for a programming check.
Do you see changes in patients’ aided or implanted detection scores?
No. I have not seen large fluctuations in that regard, unless the internal device is moving or migrating in an unexpected way. I do not see a huge change in performance, typically.
What do you tell parents with babies of newly diagnosed hearing loss when they ask if it is okay to fly internationally? I diagnose a lot of babies who fly back to China, sometimes days after I see them, and they do not have a chance to see an ENT or have imaging done.
That is a tough situation. I would provide them information and let them know that in some instances, hearing can fluctuate, whether it be from Eustachian tube dysfunction when you are flying that long, or potentially due to more serious causes like EVA. That is a hard predicament to put yourself in. I think providing the information is important, even though it can be overwhelming to do all of that in one appointment. We have to stress that they follow up once they are overseas and make sure that imaging is done. I do not know if I would necessarily tell them not to fly, but if they have the chance to see an ENT while they are in the States, that may be the way to go.
References
Gopen, Q., Zhou, G., Whittemore, K., & Kenna, M. (2011). Enlarged vestibular aqueduct: review of controversial aspects. The Laryngoscope, 121(9), 1971-1978. doi: 10.1002/lary.22083
Madden, C., Halstead, M., Benton, C., Greinwald, J., & Choo, D. (2003). Enlarged vestibular aqueduct syndrome in the pediatric population. Otology & Neurotology, 24(4), 625-632.
Merchant, S. N., Nakajima, H. H., Halpin, C., Nadol, J. B., Lee, D. J., Innis, W. P., et al. (2007). Clinical investigation and mechanism of air-bone gaps in large vestibular aqueduct syndrome. The Annals of Otology, Rhinology, and Laryngology, 116(7), 532-541.
Merchant, S. N., & Rosowski, J. J. (2008). Conductive hearing loss caused by third-window lesions of the inner ear. Otology & Neurotology, 29(3), 282-289. doi: 10.1097/mao.0b013e318161ab24
Pyle, M. G. (2000). Embryological development and large vestibular aqueduct syndrome. The Laryngoscope, 110(11), 1837-1842. doi: 10.1097/00005537-200011000-00014
Valvassori, G. E., & Clemis, J. D. (1978). The large vestibular aqueduct syndrome. The Laryngoscope, 88, 723-739.
Cite this Content as:
Wolf, J. (2015, October). Advanced management of complex cases: Enlarged vestibular aqueduct. AudiologyOnline, Article 15571. Retrieved from https://www.audiologyonline.com
