Learning Outcomes
After this course, participants will be able to:
- Identify the main components of the ADHEAR audio processor.
- Describe the correct placement and typical longevity of the ADHEAR adhesive adapter.
- Describe the four key advantages of the ADHEAR system.
- Describe candidacy for MED-ELs ADHEAR system.
Introduction and Overview
This course will be a basic introduction to this new option for your patients. I recognize that some of the participants in this course may have a lot of experience working with MED-EL over the years. Others among you may be somewhat new to MED-EL as a company. I thought I'd take a few minutes to give you an overview of our mission and our company. MED-EL's mission is to overcome hearing loss as a barrier to communication and quality of life. We effectively yet gently restore hearing by offering a comprehensive set of intact-skin hearing solutions. We thus provide the best benefit to individuals with a hearing loss, today, tomorrow, and worldwide.
MED-EL is a global company, present in 118 countries around the world. Our headquarters are located in Innsbruck, Austria, where we continue to grow. In the United States, we just opened a new building in Durham, North Carolina. We are a privately held company. Dr. Ingeborg Hochmair is the CEO and chief engineer of MED-EL. She founded MED-EL with her husband, Dr. Irwin Hochmair. From the 1970s, when their research and early implants started, to the hiring of MED-EL's first employees in 1990, we have grown and contributed to the outcomes and the greater understanding of hearing loss and the restoration of a sense via the cochlear implant, although cochlear implants are not our only focus. We have other areas of research and development in hearing science, vestibular science, and other medical areas.
Who Uses Bone Conduction (BC) Hearing Devices?
Typically, those using bone conduction devices have a conductive hearing loss. However, many bone conduction devices are also used and are suitable for mixed hearing losses and single-sided deafness. There are many bone conduction device options available on the market, some of which involve surgery. These BC devices fall into two main categories: direct drive (i.e., the mechanical vibration is direct to the bone) and skin drive (i.e., the vibration must pass through the skin to get to the bones of the skull).
Within direct drive, we have what are called active transcutaneous devices. This means that the transducer is fully implanted under the skin and is directly driving the bone. There is only one example of this type of technology. It has been available since 2012, but it is not approved in the United States at the time of this recording. The key here is intact skin, and the mechanical vibrations emanating directly from that implanted portion. Also within the direct drive category, we have the percutaneous devices. These are familiar to us as the Oticon Medical Ponto and the Cochlear Baha. These are abutment-based devices.
Within the skin drive category, we have the passive nonmagnetic devices. This category encompasses all of our conventional devices such as headbands, soft bands, and bone conduction eyeglasses. Also within the skin drive category are the passive transcutaneous systems. These would involve a surgery in order to implant a magnet. Examples of passive transcutaneous systems include the Sophono Device and the Baha Attract.
Challenges of Conventional Non-Surgical BC Devices
I would like to focus on the conventional options, specifically the softband options that have been available since the early 2000s (softbands, hardbands, and bone conduction eyeglasses). They've been used by individuals who are either not ready for surgery, unwilling to undergo surgery, or perhaps are unsuitable for surgery. When they're worn, they've been generally effective in providing audibility, but they're not without some drawbacks. I'd like to highlight a few of the challenges that are faced by users, caregivers, and audiologists alike in this particular category.
Discomfort. For the most part, when softbands are worn, they work. But what do we find? The first issue is the wearing of a softband requires some degree of pressure against the head for sound transmission. The result of that pressure against the head is discomfort. It's not an enjoyable wearing experience. In fact, it can cause pressure points and even headaches at times. Why does this matter to you or your patient? The result is a shorter wear time and some degree of user dissatisfaction.
Reliability issues. We see reliability issues, partly due to the discomfort and partly due to retention of the softband itself. These softband solutions are moved around on the head throughout the day. Sometimes they slip or slide. Most of the time, it appears they're not in the best position for the user. As such, when you are doing aided testing or working with your patient in the sound booth, make sure that the device is placed in the best position possible for that testing. In the real world, that system is going to be quite inconsistent in its placement. In addition, even when they're in the best possible position, they're not placed as close to the cochlear as would be optimal for sound transmission. Adding another layer to this puzzle, most often they are placed over the hair, causing some hair-related attenuation. The outcome is inconsistent sound quality throughout the wearing time, which may impact user satisfaction and compliance with your recommendations.
Poor cosmetic appeal. The need to wear a headband or a softband in combination with a fairly high profile device attached to it has implications. Certainly, very few adults will opt for a softband solution. Even young children can be quite self-conscious about these systems. They're highly visible. The high-profile sound processors attach to a coupling plate, and it makes for even poorer aesthetics.
The impact of those three issues -- discomfort, reliability issues, and poor cosmetic appeal -- results in limited daily wear time. Some families do the best they can for as long as they can, but frankly, they're often waiting until their child reaches that age of five where they're eligible for a bone conduction implant. They're not delighted; they're not satisfied with the status quo. Some patients simply walk away. I know that this certainly happens a lot with adults and even children. If they're not ready for a surgical option or a bone conduction implant, they are unlikely to commit to a softband solution, and they may be lost to follow-up. Unfortunately, until recently, it had been years since we've seen a major innovation in non-surgical bone conduction technology.
ADHEAR: A Better Non-Surgical Option
At MED-EL, we recognized a clear need for a better non-surgical system, one that's safe and effective, that's reliable, and provides consistent hearing for patients with conductive hearing loss. We would like it to be easy, more comfortable, more reliable, have a better aesthetic, and be suitable for a broad age range. With that identified need, we are delighted and proud to introduce ADHEAR. It is comfortably worn all day long. Its placement is reliable. The audio processor is low-profile and cosmetically appealing. It's easy to use and easy to wear. It doesn't require surgery. It's also easy to dispense. Additionally, ADHEAR is suitable for a broad age range.
For clinicians, parents, and patients, ADHEAR repeats a revolution in bone conduction technology. With ADHEAR, the users will appreciate an easier management process, unparalleled wearing comfort, reliable placement, good retention, improved cosmetics, and a device that's enjoyable to wear. ADHEAR represents a category of bone conduction devices redefined.
Let's take a moment to watch a video which will review how ADHEAR works. After the video, I will provide more specific information on the system.
ADHEAR External Landmarks
The ADHEAR Audio Processor System is available in three colors: Dove Silver, Terra Brown, and Simply Black. The adhesive adapters, which are new to all of us, are available in two colors: beige and brown. Let's walk through the basic external landmarks of the device just to familiarize you with the system.
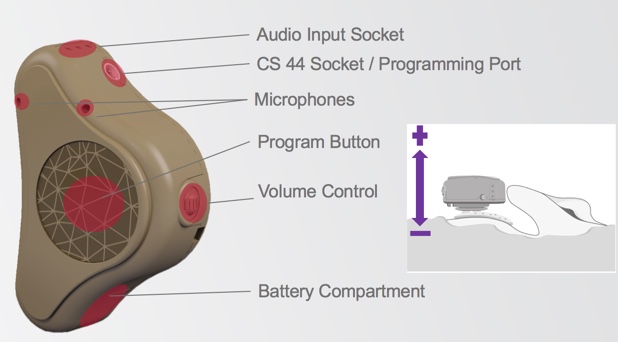
Figure 1. ADHEAR basic features.
In Figure 1, we can see the dual microphones, the large program button, and the scrolling volume wheel. When you use this volume control wheel, scrolling it away from the skull increases the volume, and scrolling it in towards the skull decreases the volume. We can also see the battery compartment, the audio input socket, and the programming port, which takes a CS 44 cable.
When we turn the device over (Figure 2), you can now see the retention clip fixture, the serial number, and the coupling plate which connects to the adhesive adapter.
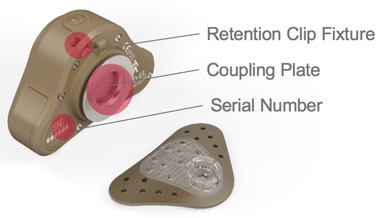
Figure 2. ADHEAR basic features.
The main landmarks of the adhesive adapter are the snap connector to which the coupling plate connects, and then the protective film (Figure 3).

Figure 3. Snap connector and protective film.
The adhesive adapters are single use only. Once you've applied it to the skin, you cannot remove it and reapply. The adhesion will not work for reapplication. In Figure 4, you can see the adhesive once the protective film has been removed.

Figure 4. Adhesive once the protective film has been removed.
The device has a symmetrical design (i.e., the system can be used on either ear). There's no difference for you as an audiologist in the ordering process, nor in the configuration or software process. The volume control wheel will work in the same way whether it's on the right or left side. Again, scrolling outwards or away from the head increases the volume, and scrolling inwards or towards the head decreases the volume. In addition, the front microphone is always in front, whether it is placed on the right side or the left side.
The system ships to you preset and optimized for the narrow fitting range of a conductive hearing loss. Using the configuration software, you can make changes as desired. That's something I will cover in much more detail in the AudiologyOnline session named Fitting ADHEAR on Your Patients. The volume control is enabled, and four programs are available:
- P1: Fully automatic, NR, FBC
- P2: Omni, NR, FBC
- P3: Omni, NR off, FBC off; or DAI 50:50
- P4: Mute mics; or DAI
They are in a logical order with a logical series of signal processing features that would allow easy use of the device for many of your patients and easy demonstration of the system for you as a clinician. As I mentioned, if you have a desire to limit access to the volume control or turn on or off certain programs, you can do so with the configuration software.
ADHEAR: A Revolution in BC Technology
ADHEAR is a revolution in bone conduction technology. It's a completely new kind of bone conductor that overthrows two major dogmas in bone conduction technology. The first is the ability to achieve bone conduction without pressure. Efficient bone conduction stimulation without pressure against the head and without surgery is now possible. With ADHEAR, your patients will be able to wear their device all day long, without any discomfort, without pressure issues.
The second revolutionary aspect is the proprietary patented adhesive adapter. The development of this dual interface adhesive unit has enabled us to have our patients wear this system attached to the same spot on the skin day after day, week after week, month after month, without irritating the skin. Again, this is something that was not thought to be possible but that we have made possible with ADHEAR.
No Pressure
In the softband condition, we are not competing against perfect systems. Those systems lose efficiency in several ways, but with ADHEAR, we win back efficiency in three critical ways:
- By minimizing the mass of the skin contact plate
- By optimizing the position of the system
- By placing the adhesive adapter on the hairless area of the mastoid
Minimizing the mass of the skin contact plate. This maximizes the efficiency of sound energy that is transferred to the bone. A conventional bone conduction system that uses a softband is attaching the audio processor to a skin contact plate. That audio processor first must vibrate the mass of that contact plate and the headband system in order to send sound energy through to the skull. The mass of that contact plate is high in the existing systems, relative to the audio processor. They lose efficiency in this process. With ADHEAR, by reinventing the skin contact plate as a dual interface adhesive adapter weighing less than one gram, we enable as efficient a sound transfer as possible between the device and the head.
Optimizing the position of the system. We're placing ADHEAR at position B for optimal stimulation of the ipsilateral cochlea (Figure 5). Remembering back to graduate school, we were taught that interaural attenuation is zero dB for bone conduction; as such, maybe it doesn't matter where you place the device on the head. However, it is important to know and recognize that this is highly variable from person to person, and it is variable across the frequency range. The optimal position for conductive hearing loss where we are trying to stimulate the ipsilateral cochlea is as close as possible to that cochlea. Position A is where most softbands sit when they're in the best position they can be, usually when you're doing your aided testing. Experience certainly tells us that they don't reside there all day long; they often sit elsewhere in the head. That could be due to discomfort or slippage, retention, et cetera. This is the second way the competitive softband devices lose efficiency in sound transmission. With ADHEAR, the optimal position on the mastoid is maintained consistently by the adhesive adapter.
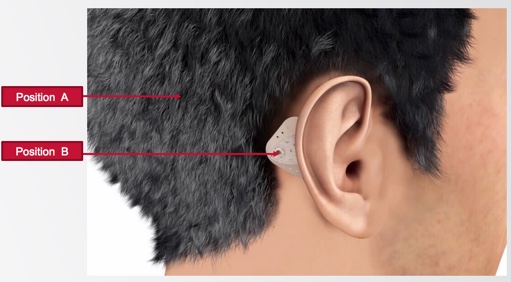
Figure 5. Optimal position behind the ear.
Adhering to the hairless area. By placing the adhesive adapter on the hairless area of the mastoid, ADHEAR does not have challenges related to hair-related attenuation. This is in contrast to conventional softband systems, which typically sit over the hair.
In summary, we maximize sound energy transfer to the bone by minimizing the mass of the skin contact plate. In addition, the placement of the adhesive adapter is optimal for stimulation of the ipsilateral cochlea. Finally, we're placing it on a hairless zone so we do not suffer from hair-related attenuation. With ADHEAR, efficient bone conduction stimulation is possible without pressure against the skin and without surgery, so your patient will be able to wear the device all day long.
Dual-Interface Adhesive Adapter
Now, let's take a look at the second revolutionary aspect of ADHEAR: the proprietary patented Dual-interface Adhesive Adapter. The development of this adhesive unit has enabled us to have our patients wear the device and attach to that same optimal position on the skin, day after day, week after week, month after month, year after year. This is possible due to the right biocompatible materials, the right strength of the adhesive, and even a change interval for the adhesive adapters that complements and works with the skin and the renewal rate of the skin cells.
The adhesive adapter has two interfaces: the interface between the skin and the adapter, and between the adapter and the device. Both are optimized for a pleasant and easy user experience.
Interface between the skin and the adapter. In developing the skin adapter interface, we needed to develop and select an adhesive strength that was capable of allowing the patient or the user to repeatedly attach and remove the audio processor without dislodging the adhesive adapter from the skin. To be clear, when your patient places the adhesive adapter, they will wear that adhesive adapter for three to seven days. They'll get up in the morning, place their device. They'll remove the device at any point during the day or at night and replace it the next day. That repeat connection and removal is possible without dislodging the adhesive adapter. This required the selection of an adhesive strength that was capable of lasting a reasonable timeframe. We wanted the adhesive longevity to complement the magnitude of the skin renewal rate because if our patients need to change it too often, it would be difficult for the skin to tolerate removal and replacement. It's important to note that we use a medical grade adhesive. It's non-sensitizing and can be worn in the same spot on the skin for repeat replacements day after day without irritation. It's also water compatible. Certainly, the audio processor is not waterproof, but the user can take showers or go swimming wearing the adapter, and it will be fine. We do suggest that when users jump out of the shower that they pat down the adhesive adapter to reduce any water content.
Interface between the audio processor and the adhesive adapter. The adapter-device interface also involved important innovation and development designed for the durability of the device. In contrast with competitive devices, we place the wear and tear on the plastic snap connector of the adhesive adapter. The coupling plate on the audio processor itself is designed to last. Why does this contrast with our competition? It's because conventional softband devices are also designed for use within an abutment. In those cases, users need the wear and tear to be on the device rather than on the abutment, because the abutment is fixed and cannot be repaired. Whereas, with ADHEAR, we can have the wear and tear on our adhesive adapter, because it is designed for short life (three to seven days). The durability requirements on that snap connector need to be limited to about two weeks. This also allows us the pleasure of a low profile arrangement, which enhances the cosmetic appeal. Competitive devices (designed for use with abutments) don't know exactly where the skin level will be. With ADHEAR, we know exactly where the skin level is because this is a non-surgical system that does not require an abutment. The result is when worn, this device simply does not stick out as much on the head as devices on softbands.
In summary, with regard to the long-term use of an adhesive adapter, it is designed to stay in place when attaching and replacing the audio processor. The adhesive strength and longevity match the skin renewal rate, and the adhesive is medical grade and non-sensitizing. Your patients will be able to wear this device all day long with comfort and ease.
We do have early usage data from Birmingham, Warsaw, and Antwerp. That feedback is showing that ADHEAR is a comfortable system, easy to use, well accepted by children and parents, and causes no soft tissue reaction. We'll discuss our research findings further in a presentation on AudiologyOnline, and you'll see some papers published over the coming months and years. At this point, what we're seeing is that ADHEAR performs at least as well as softband devices using a size 13 battery. Even without pressure, we've developed a reliable method of bone conduction sound transmission. In doing so, we have revolutionized the cosmetics, the comfort, and the ease of non-surgical bone conduction technology.
Steps to Positioning and Using ADHEAR
Now we're going to get practical. Although the process of positioning and using ADHEAR is easy, since it's a new concept to everyone participating in this course, I'm going to walk through successful positioning step by step. If you're a clinician, I would recommend practicing on yourself or on a colleague a few times before working through this with patients. From what we've seen in our research sites, if you ensure that patients have been trained on the application of the adhesive adapter and on the removal of the audio processor, there are high levels of success with the system.
This is a picture of our optional positioning tool (Figure 6). For your initial placements of ADHEAR, this optional positioning tool may be helpful in a couple of ways. The first would be to give you a good visual and help you identify the optimal positioning behind the ear for that adhesive adapter. Secondly, you can use it to place the adhesive adapter.
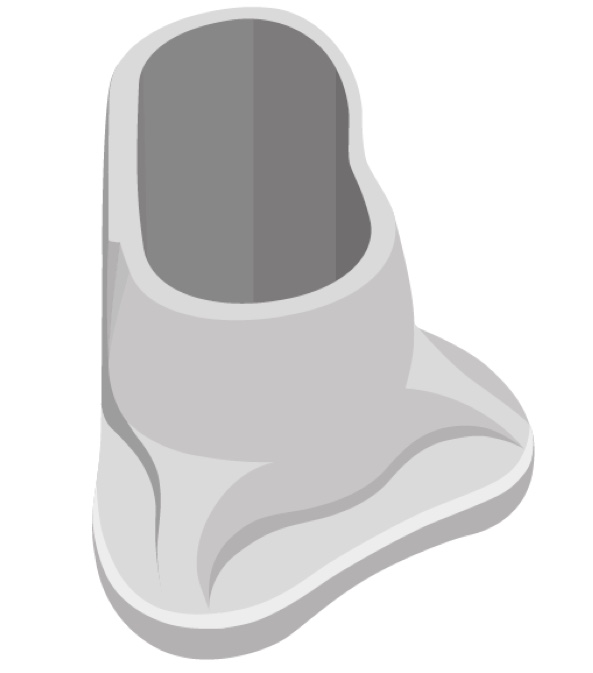
Figure 6. Optional positioning tool.
When positioning ADHEAR, there are three key things to do to prepare the site:
- First, make sure that the site is clean. You can use soap and water or an alcohol wipe to clean the area.
- Second, make sure that the site is allowed to dry thoroughly. If you're in a humid climate, or if a child or adult is perspiring, give them a few minutes to cool down.
- Third, make sure the site is hairless. The area behind the ear is usually adequate in terms of size (i.e., it's a hairless zone that is large enough to accommodate the adhesive adapter). If necessary, you may need to adjust the hairline by shaving it.
Once the site is prepared, hold the positioning tool in the appropriate position on the mastoid behind the ear (Figure 7). If you're working with a typically developed pinna, the tool should likely touch the ear. If desired, you can use a marker to outline where you'll put the adhesive. That might be helpful for family members as they get some practice doing this. You can use the positioning tool as your guide.
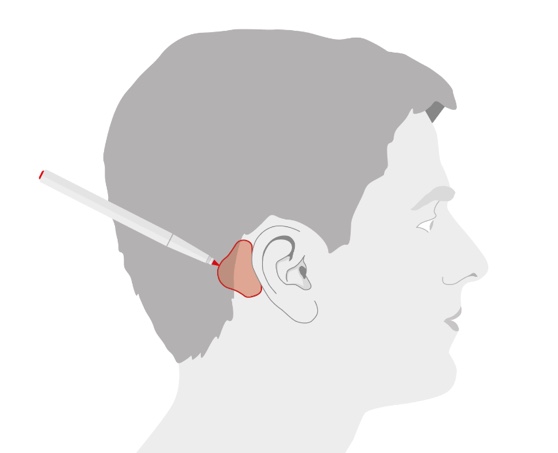
Figure 7. Optimal position for the adhesive adapter.
From here, you can place the adhesive adapter directly, by removing the protective film and placing it appropriately on that outlined area. Or, if you wanted to use that positioning tool to assist you in the positioning, you certainly could do that. You would first place the adhesive adapter in the cavity, remove the protective film, and then place it behind the ear. Once it's behind the ear, you would press down on the positioning tool firmly so that it's lightly touching the ear. Press down on the superior and inferior aspects of the tool and remove it gently. At that point, the adhesive adapter is in position (Figure 8). Then press firmly on the adhesive adapter with your fingers. The adhesive adapter is in place at that time. If the site is clean, dry and hairless, you'll have excellent adhesion with the adhesive adapter.
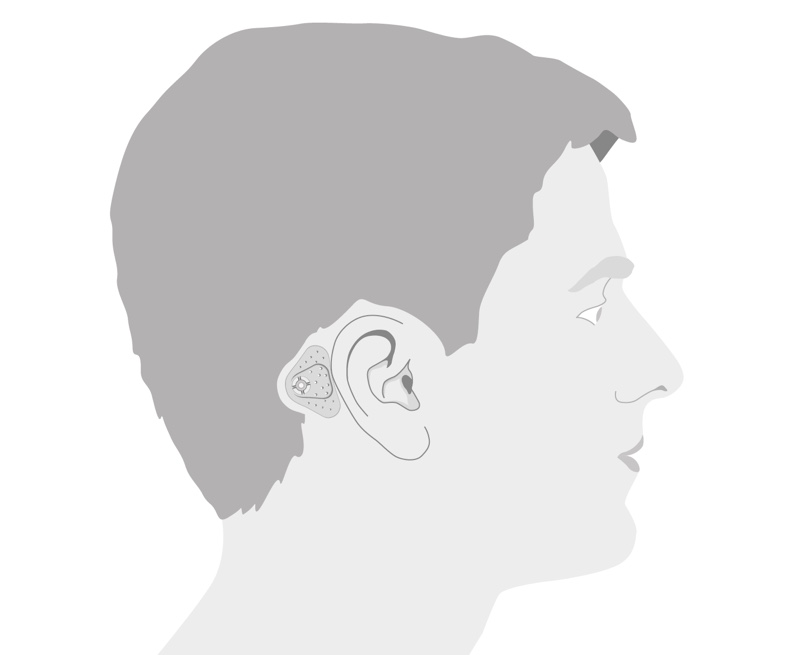
Figure 8. Successful placement of adhesive adapter.
Coupling ADHEAR to the Adhesive Adapter
Now that you have your adhesive adapter in place, it's time to attach the audio processor to the adapter. The ADHEAR takes a size 13 battery. I'd suggest that you go ahead and insert the battery. You might want to partially close that battery door so that feedback is not occurring while you're attaching the audio processor to the adhesive adapter. You will then find the coupling position. In Figure 9, you can see that from behind the ear, you tilt the audio processor at a slight angle onto that snap connector.
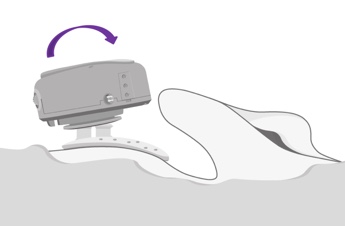
Figure 9. Coupling ADHEAR to the adhesive adapter.
Once it's attached to the snap connector, if you feel that the audio processor needs any adjusting, you can easily press and rotate the audio processor for slight adjustments. It is important to point out that the audio processor should not touch the pinna when it is in place, because otherwise, you're likely to have challenges with feedback. Figure 10 shows the correct positioning of ADHEAR.
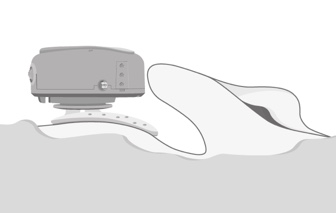
Figure 10. Correct positioning of ADHEAR.
Detaching ADHEAR
At the end of the day, your patient will want to remove the ADHEAR at bedtime. If he or she wishes to disconnect the audio processor from the adhesive adapter, they should press the audio processor down at the side that is closest to the pinna, or to the ear (Figure 11). You don't want to pull it off the head, and you don't want to press down on the rear, as either of those two actions may dislodge the adhesive adapter from the skin. Reminding and having your patient practice this when you're dispensing is key to success.
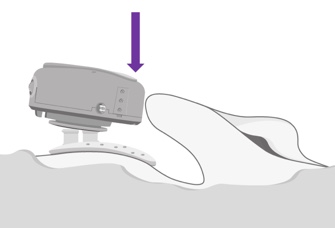
Figure 11. Detaching ADHEAR.
Although it takes a bit of practice, using and handling the ADHEAR system is easy, as demonstrated in the following video.
Summary
As you adjust to this new method of coupling a hearing device to your patient, keep the following in mind:
- ADHEAR adhesive adapters are designed to be worn for three to seven days, depending on each patient's frequency of change rate, on their activity level, skin type, etc.
- ADHEAR adhesive adapters should be stored at room temperature. Each pack of adhesive adapters comes with a use-by-date.
- ADHEAR adhesive adapters come in packs of 15 and are available in two colors: beige and brown.
- ADHEAR adhesive adapters are water compatible. They do not need to be removed when showering or bathing.
- When changing adapters, they should be removed in the evening, and reapplied the next morning.
Candidacy for ADHEAR
Earlier, we discussed the general candidacy for bone conduction hearing devices. Now, we will review ADHEAR's specific candidacy in the context of conductive hearing loss. ADHEAR is suitable for pediatric and adult patients who fit within the candidacy and indication criteria, with unilateral or bilateral conductive hearing loss, either chronic or temporary. Bone conduction thresholds should be within the range indicated in Figure 12. This technology is better suited for conductive losses rather than mixed losses.
Figure 12. Candidacy for ADHEAR.
As you start to think about your patients, who may benefit from ADHEAR? When it comes to age, ADHEAR is suitable for a broad age range, provided the individual fits the indication and candidacy range. Of course, conductive hearing loss may occur at any age. It's known that the most common cause of intermittent mild to moderate acquired hearing loss in children is conductive hearing loss associated with otitis media, which varies in prevalence. Across the globe and even across the country, factors like population size, socioeconomics, even access to healthcare, all play into the variance that we see in prevalence. Other causes of conductive hearing loss can include oral atresia, poor eustachian tube function, perforated tympanic membrane, tumors, head trauma, otosclerosis, and otitis externa. Conductive hearing loss can occur at any age and has a broad range of etiologies. Some conditions are temporary in nature; others are medically or surgically treatable over a period of time.
Under the age of five in the U.S., children are not approved for bone conduction implants. This group is considered unsuitable for surgery. Over the age of five, surgical candidacy may be related to the health and readiness of the individual. Of course, there may be other medical or surgical considerations. An example of this is a patient with cranial facial conditions. For these individuals, the timing of the bone conduction implant surgery may need to align with a series of more complicated surgeries involved in reconstruction. The great news is that with ADHEAR, not only do you have no pressure from the device, you also have no pressure to move or rush towards a surgical decision until the time is right for your patient and your patient's family.
Four Key Advantages of ADHEAR
As we wrap up this presentation, I want to highlight four key advantages for your patients using the ADHEAR system.
- ADHEAR is effortless. It's a user-friendly device. It offers easy handling. It's easy to maintain. You're not going to have any complaints of headband head or difficulties finding headbands or bows to match different outfits. In addition, it is a non-surgical option.
- ADHEAR is gentle. The unique development of the adhesive adapter is a true innovation in bone conduction technology. There is no pressure on the skin, even when the system is in place, and the audio processor is coupled to the adhesive adapter. At a weight of less than 15 grams, including the battery, it is very light.
- ADHEAR is reliable. Knowing that the positioning and the design of the adapter and the audio processor will result in a consistent and reliable placement of the device relative to the cochlea is a great relief for parents and patients and clinicians, resulting in consistent access to sound throughout the day.
- ADHEAR is cosmetically appealing. The device offers aesthetics that are superior to the alternatives that are currently available. In addition to the fact that we don't have to worry about the softband requirement, it is low profile and fits inconspicuously behind the ear. It can be individualized using silicone sleeves and fun stickers (Figure 13).

Figure 13. Individualizing ADHEAR.
Summary and Conclusion
We are hopeful that ADHEAR will be attractive across a broad range of organizations. Pediatric audiology centers will likely consider ADHEAR as a viable option for their patients. ENT clinics see patients every day who fit that candidacy range, and audiologists are looking for alternatives for these patients. School systems may use ADHEAR with children presenting to school with permanent or temporary conductive hearing losses. Audiology centers who work with craniofacial teams will enjoy having another option in their toolkit. Even non-medically-based private practices may have patient groups who are candidates for non-surgical bone conduction systems. These audiologists may be able to cooperate with medical teams, or larger tertiary centers and hospitals, to provide local services to candidates.
We also have our in-house audiology team ready to assist with any first fittings, any basic software support or training about the device. You can also reach out to your local MED-EL representative. Across the nation, we'll be hosting regional training events this year. Please do contact us to find out if we have a regional training event coming up in your area. In terms of your patients, they'll find that our website is very user-friendly. I think it's great for both professionals and patients. They can also consider accessing the Worldwide HearPeers Forum, and that might be a good place to connect with other candidates and recipients. Thank you for your participation today. We hope you'll feel comfortable reaching out for us for any support that you might need in incorporating ADHEAR as an option in your practice.
Citation
O'Donnell, A. (2018). ADHEAR - A revolution in bone conduction technology. AudiologyOnline, Article 23691. Retrieved from https://www.audiologyonline.com


