ROCKEFELLER UNIVERSITY PRESS - A team of New York-based researchers has compared the effects of two disease-causing mutations, potentially explaining why patients with the rare genetic disorder keratitis-ichthyosis-deafness (KID) syndrome can experience different sets of symptoms. The study, "Syndromic deafness mutations at Asn 14 differentially alter open stability of Cx26 hemichannels," will be published online June 27, 2016 in The Journal of General Physiology.
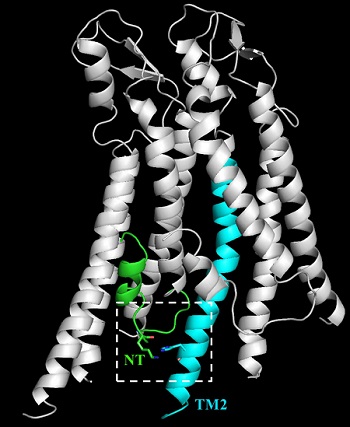
The X-ray crystallographic structure of two Cx26 subunits suggests that the N14K mutation in the protein's N-terminal domain (green) may stabilize the hemichannel's open state by interacting with a transmembrane domain (blue) of a neighboring subunit. Sanchez et al., 2016.
Connexin proteins form "hemichannels" in the plasma membrane of cells that allow ions and small molecules to pass between a cell and its surroundings, or between two neighboring cells, if the hemichannels of both cells are aligned. Mutations in Connexin 26 (Cx26) cause KID syndrome, which is associated with profound deafness, visual problems, and skin abnormalities. Patients carrying the N14Y mutation, in which an asparagine residue in Cx26's N-terminal domain is mutated to tyrosine, experience all these symptoms. But patients carrying the N14K mutation, in which the same asparagine residue is changed to lysine, experience a different set of symptoms. These patients do not suffer vision loss, but they do develop problems in various mucosal tissues, such as the lips, gums, and lining of the tongue.
To understand why the N14Y and N14K mutations have distinct effects in patients, Helmuth Sanchez and Vytas Verselis, from the Albert Einstein College of Medicine, together with Nefeli Slavi and Miduturu Srinavas from the SUNY College of Optometry, expressed the mutant proteins in cells and investigated how they affected the properties of Cx26 hemichannels.
Hemichannels containing the N14Y mutation showed much lower ion conductance than normal, wild-type hemichannels, perhaps because the mutation destabilizes the channel's open, ion-conducting, state. N14K, in contrast, appeared to stabilize hemichannels in their open state, thereby allowing robust ion conductance. Wild-type Cx26 channels close in response to a reduction in pH, but N14K-containing channels remained open at a variety of pH levels.
"The effects of hemichannel opening would therefore be exacerbated in acidic environments, as seen in many mucosal tissues," says Vytas Verselis. "This might explain the unusual mucocutaneous manifestations described in patients carrying the N14K mutation."
The researchers nevertheless want to investigate other potential effects of the N14Y and N14K mutations, determining, for example, how they affect the passage of ions and small molecules between cells, and whether they alter the expression or localization of Cx26 channels in patient tissues.
Sanchez, H., et al. 2016. J Gen. Physiol. https://dx.
About The Journal of General Physiology
The Journal of General Physiology (JGP) features peer-reviewed research in biological, chemical, or physical mechanisms of broad physiological significance, with an emphasis on physiological problems at the cellular and molecular level. All editorial decisions are made by research-active scientists in conjunction with in-house scientific editors. JGP provides free online access to many article types immediately, with complete archival content freely available online. Established in 1918, JGP is published by The Rockefeller University Press. For more information, visit jgp.org.
Source: https://www.eurekalert.org/pub_releases/2016-06/rup-wks062716.php

