Durham, NC - (January 31, 2007) -MED-EL announced today the commercial availability of the Soundbridge® middle ear implant in the United States. The Soundbridge is the first U.S. Food and Drug Administration (FDA) approved implantable middle ear hearing device to treat sensorineural hearing loss. A proven, safe and effective treatment that leaves the ear canal completely open, the Soundbridge features a 94 percent improvement in patient satisfaction,1 with thousands of patients worldwide.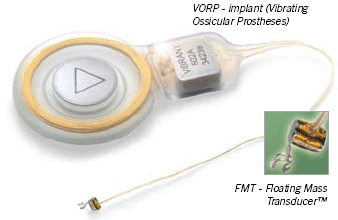
The Soundbridge is indicated for use in adults who have moderate-to-severe sensorineural hearing loss and desire an alternative to an acoustic hearing aid. The product utilizes hearing technology that directly drives the ossicular chain (middle ear bones), bypassing the ear canal and tympanic membrane (eardrum). It consists of two major components:
- the implanted component, called the Vibrating Ossicular Prosthesis™ (VORP™), and
- the externally-worn receiver, called the Audio Processor™ (AP), which is approximately the size of a quarter.
Unlike a hearing aid, which simply amplifies sound, the Soundbridge is a direct drive prosthetic, which mechanically vibrates the bones in the middle ear without surgically altering the structures of the middle ear.
"The Soundbridge overcomes inconveniences of traditional in-the-ear hearing aids, such as distortion and ear canal irritation," said Fredrick Lassen, MD, of the Lakeview Medical Center in Suffolk, VA. "A lot of people with hearing loss, particularly gradual onset hearing loss, don't understand all of the options available to them. It's exciting to be able to discuss this option with patients who are dissatisfied with their conventional hearing aid." Dr. Lassen was the first surgeon in the United States to conduct a Soundbridge middle ear implant surgery after the product's initial FDA approval.
"We're pleased to provide this exciting option to the millions of Americans who suffer from moderate-to-severe sensorineural hearing loss," said Richard Collette, CEO of MED-EL North America. "The Soundbridge is an important addition to our technologically-advanced line of products, broadening the spectrum of choices for people and improving quality of life for those with hearing loss."
People who participated in the Soundbridge clinical trial reported improved sound clarity, better fit and comfort, increased gain (loudness of sound), and fewer maintenance issues than they experienced with hearing aids. In addition, study participants reported statistically significant improvement in their ability to hear and communicate while using the Soundbridge in a variety of difficult listening situations, such as: improved ability to hear familiar talkers, increased ease of communication, better performance in environments with high levels of reverberation or background noise, less distortion of sound, better performance when other listening cues were reduced, and fewer uncomfortable or unpleasant sounds.
And, one of the benefits of using the Soundbridge is that it did not significantly impact residual hearing in 96% of patients, which provides a measure of comfort to people who are dissatisfied with hearing aids but wary of a surgical procedure.2
About Hearing Loss in the United States
Hearing loss has a profound impact on the everyday lives and pocketbooks of Americans. Approximately 31.5 million Americans - or 1 in 10 - suffer from some kind of hearing loss.3 The most common form of hearing loss is sensorineural, which affects an estimated 60 percent of those with hearing loss. This involves damage to the inner ear caused by aging, pre-natal and birth-related problems, viral and bacterial infections, heredity, trauma, exposure to loud noise, as well as a significant number of unknown reasons.
Research has shown that only 1 out of 5 people who could benefit from a hearing aid actually wear one.4 For America's 24 million people with hearing impairment who do not use hearing instruments, the estimated cost in lost earnings due to untreated hearing loss is $122 billion annually. At a 15 percent tax bracket, the cost to society could be well in excess of $18 billion due to unrealized taxes.5 For those with hearing loss that do use hearing aids, more than 9 out of 10 (93 percent) indicate that their quality of life has been positively impacted by their hearing instrument usage at least "some of the time." Yet only half (50 percent) say their social life, ability to join in groups, relationships at home, feelings and confidence in self, sense of safety, and relationships at work have improved due to hearing instruments.
About MED-EL
In June 2003, MED-EL signed an acquisition agreement for the Vibrant Soundbridge® with technology developer Symphonix. Geoffrey Ball, Symphonix founder and inventor of the Vibrant Soundbridge, who himself is implanted bilaterally with the system, joined the MED-EL team as technical director for the Soundbridge. MED-EL has recently integrated the Soundbridge into its cochlear implant manufacturing and quality control process in Innsbruck, Austria.
Since its founders developed one of the world's first cochlear implants in 1975, MED-EL has set new standards in hearing implant technologies, developing and manufacturing technologically advanced hearing solutions for people with varying degrees of hearing loss. MED-EL hearing implant systems, currently used in 80 countries, combine the latest scientific advances, engineering and manufacturing techniques for performance, safety and reliability.
MED-EL currently supports Soundbridge® users in the United States as well as services the device.
Footnotes
- Luetje C, et al. "Phase III Clinical Trial results with the Vibrant Soundbridge Implantable Middle Ear Hearing Device.Prospective Controlled Multicenter Study." Otolaryngology-Head & Neck Surgery 2002; 126; 2:97-107.
- Luetje C, et al. "Phase III Clinical Trial results with the Vibrant Soundbridge Implantable Middle Ear Hearing Device.Prospective Controlled Multicenter Study." Otolaryngology-Head & Neck Surgery 2002; 126; 2:97-107.
- Kochkin, S. "MarkeTrak VII: Hearing Loss Population Tops 31 Million," The Hearing Review, July 2005.
- National Institute on Deafness and Other Communication Disorders; www.nidcd.nih.gov/health/statistics/hearing.asp
- Kochkin, S. "The Impact of Untreated Hearing loss on Household Income," August 2005, Better Hearing Institute, Alexandria, VA.
Taken from www.vibrantmedel.us/archive/layout/MEDEL_PR_Soundbridge.pdf

