U-M-led team shows key role of protein called NT3 in ear-to-brain communication, and as a target for future treatments.
ANN ARBOR, Mich. — Scientists have restored the hearing of mice partly deafened by noise, using advanced tools to boost the production of a key protein in their ears.
By demonstrating the importance of the protein, called NT3, in maintaining communication between the ears and brain, these new findings pave the way for research in humans that could improve treatment of hearing loss caused by noise exposure and normal aging.
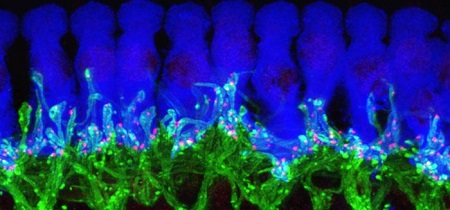
This microscope image of tissue from deep inside a normal mouse ear shows how ribbon synapses (red) form the connections between the hair cells of the inner ear (blue) and the tips of nerve cells (green) that connect to the brain. Credit: Corfas lab - University of Michigan.
In a new paper in the online journal eLife, the team from the University of Michigan Medical School's Kresge Hearing Research Institute and Harvard University report the results of their work to understand NT3's role in the inner ear, and the impact of increased NT3 production on hearing after a noise exposure.
Their work also illustrates the key role of cells that have traditionally been seen as the "supporting actors" of the ear-brain connection. Called supporting cells, they form a physical base for the hearing system's "stars": the hair cells in the ear that interact directly with the nerves that carry sound signals to the brain. This new research identifies the critical role of these supporting cells along with the NT3 molecules that they produce.
NT3 is crucial to the body's ability to form and maintain connections between hair cells and nerve cells, the researchers demonstrate. This special type of connection, called a ribbon synapse, allows extra-rapid communication of signals that travel back and forth across tiny gaps between the two types of cells.
"It has become apparent that hearing loss due to damaged ribbon synapses is a very common and challenging problem, whether it's due to noise or normal aging," says Gabriel Corfas, Ph.D., who led the team and directs the U-M institute. "We began this work 15 years ago to answer very basic questions about the inner ear, and now we have been able to restore hearing after partial deafening with noise, a common problem for people. It's very exciting."
Using a special genetic technique, the researchers made it possible for some mice to produce additional NT3 in cells of specific areas of the inner ear after they were exposed to noise loud enough to reduce hearing. Mice with extra NT3 regained their ability to hear much better than the control mice.
Now, says Corfas, his team will explore the role of NT3 in human ears, and seek drugs that might boost NT3 action or production. While the use of such drugs in humans could be several years away, the new discovery gives them a specific target to pursue.
Corfas, a professor and associate chair in the U-M Department of Otolaryngology, worked on the research with first author Guoqiang Wan, Ph.D., Maria E. Gómez-Casati, Ph.D., and others in his former institution, Harvard. Some of the authors now work with Corfas in his new U-M lab. They set out to find out how ribbon synapses – which are found only in the ear and eye – form, and what molecules are important to their formation and maintenance.
Anyone who has experienced problems making out the voice of the person next to them in a crowded room has felt the effects of reduced ribbon synapses. So has anyone who has experienced temporary reduction in hearing after going to a loud concert. The damage caused by noise – over a lifetime or just one evening – reduces the ability of hair cells to talk to the brain via ribbon synapse connections with nerve cells.
Targeted Genetics Made Discovery Possible
After determining that inner ear supporting cells supply NT3, the team turned to a technique called conditional gene recombination to see what would happen if they boosted NT3 production by the supporting cells. The approach allows scientists to activate genes in specific cells, by giving a dose of a drug that triggers the cell to "read" extra copies of a gene that had been inserted into them. For this research, the scientists activated the extra NT3 genes only into the inner ear's supporting cells.
The genes didn't turn on until the scientists wanted them to – either before or after they exposed the mice to loud noises. The scientists turned on the NT3 genes by giving a dose of the drug tamoxifen, which triggered the supporting cells to make more of the protein. Before and after this step, they tested the mice's hearing using an approach called auditory brainstem response or ABR – the same test used on humans.
The result: the mice with extra NT3 regained their hearing over a period of two weeks, and were able to hear much better than mice without the extra NT3 production. The scientists also did the same with another nerve cell growth factor, or neurotrophin, called BDNF, but did not see the same effect on hearing.
Next Steps
Now that NT3's role in making and maintaining ribbon synapses has become clear, Corfas says the next challenge is to study it in human ears, and to look for drugs that can work like NT3 does. Corfas has some drug candidates in mind, and hopes to partner with industry to look for others.
Boosting NT3 production through gene therapy in humans could also be an option, he says, but a drug-based approach would be simpler and could be administered as long as it takes to restore hearing.
Corfas notes that the mice in the study were not completely deafened, so it's not yet known if boosting NT3 activity could restore hearing that has been entirely lost. He also notes that the research may have implications for other diseases in which nerve cell connections are lost – called neurodegenerative diseases. "This brings supporting cells into the spotlight, and starts to show how much they contribute to plasticity, development and maintenance of neural connections," he says.
In addition to Corfas, Wan and Gómez-Casati, who now works in Argentina, the research was performed by Angelica R. Gigliello, and M. Charles Liberman, Ph.D. director of the Eaton-Peabody Laboratories of the Massachusetts Eye and Ear Infirmary. The research was supported by the National Institute on Deafness and Other Communication Disorders (DC004820, DC005209) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD18655), both part of the National Institutes of Health, and by the Hearing Health Foundation.
Reference: eLife, https://elifesciences.org/content/early/2014/10/17/eLife.03564
Source: https://www.eurekalert.org/pub_releases/2014-10/uomh-srh102014.php

