FDA approves MED-EL Corporation to import new PULSARCI100 August 29, 2005, Research Triangle Park, NC -- MED-EL Corporation, an implantable hearing technology firm, today introduced the revolutionary PULSAR-CI-100, which offers some of the most highly advanced cochlear implant technology available in the United States. The FDA has given MED-EL permission to begin importing the new PULSAR-CI-100 Cochlear Implant, which is already on the market in Europe and Canada. PULSAR-CI-100 is the first to incorporate I100 technology, a highly durable and power-efficient electronics platform with wafer-thin layers of sophisticated electronics arranged on a single miniaturized chip.
August 29, 2005, Research Triangle Park, NC -- MED-EL Corporation, an implantable hearing technology firm, today introduced the revolutionary PULSAR-CI-100, which offers some of the most highly advanced cochlear implant technology available in the United States. The FDA has given MED-EL permission to begin importing the new PULSAR-CI-100 Cochlear Implant, which is already on the market in Europe and Canada. PULSAR-CI-100 is the first to incorporate I100 technology, a highly durable and power-efficient electronics platform with wafer-thin layers of sophisticated electronics arranged on a single miniaturized chip.
"We have been waiting for the opportunity to offer this device to our patients because of the new, advanced electronic design and the chance to provide patients with the absolute latest in cochlear implant technology," says Dr. Pillsbury from the University of North Carolina at Chapel Hill.
Additionally, MED-EL is currently conducting research with PULSAR patients around the globe to explore a number of key concepts in cochlear implantation:*
- SmartSystemTM Research examines how technology patented by MED-EL could be used to reduce channel interaction with simultaneous stimulation.
- Fine StructureTM Research explores various approaches to providing fine frequency detail to cochlear implant users, comparing and contrasting the potential outcomes of temporal and place coding with sequential and simultaneous stimulation.
- Auditory Nerve Response Telemetry (ARTTM) Research investigates uses for patented adaptive sigma-delta modulation and precision triphasic pulses in EAP recordings.
"For over two decades, MED-EL has searched for ways to improve the understanding of speech in difficult listening environments and heighten the appreciation of music for cochlear implant users," explains Kim Jackson, Interim CEO of MED-EL Corporation. "We are confident that current and future research with the PULSAR will bring us closer to realizing our mission."
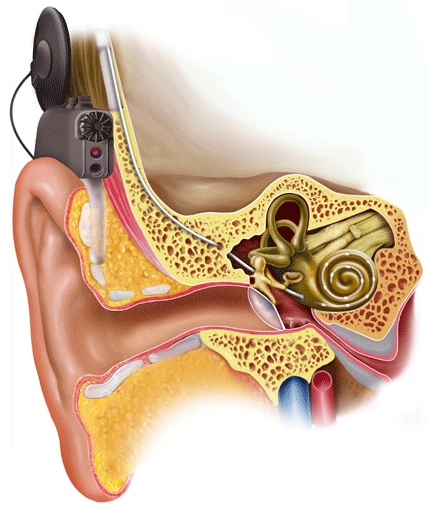
Cochlear implants were developed in the 1970's to help individuals with severe to profound hearing loss who gain little or no benefit from hearing aids. Patients wear a small speech processor behind the ear that picks up sound from the environment and transmits it to an internal component using specialized radio-frequency transmission.
About MED-EL Corporation
Since its founders developed one of the world's first cochlear implants in 1975, MED-EL has set new standards in hearing implant technologies, developing and manufacturing technologically advanced hearing solutions for people with varying degrees of hearing loss. MED-EL hearing implant systems, currently used in 70 countries, combines the latest scientific advances, engineering and manufacturing techniques for performance, safety and reliability. For more information, see www.medel.com or call 888-MED-EL-CI (633-3524).

