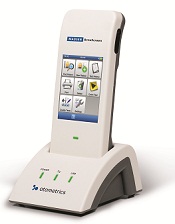
 Revolutionary Hand-Held System Improves Workflow, Eliminates Cart.
Revolutionary Hand-Held System Improves Workflow, Eliminates Cart.
Schaumburg, IL - November 06, 2012 – GN Otometrics, the world leader in hearing and balance diagnostic systems, today launched the next generation of newborn hearing screening: the new MADSEN AccuScreen two-step (OAE/ABR) hand-held screening system. The new AccuScreen, which recently received FDA clearance, is an intuitive solution for newborn hearing screening. It features a revolutionary design and interface that improves workflow efficiency and allows the user to focus on the infant. The new AccuScreen is entirely hand-held, so there is no cart required, which marks a significant advance in infant screening technology. Additionally, it is an all-in-one OAE/ABR device that is totally configurable and meets all program requirements.
New MADSEN AccuScreen: Better Workflow Efficiency Means More Focus on Infants
Because of its design and interface advances, the new AccuScreen dramatically improves workflow. The hand-held device features a completely redesigned housing that is significantly smaller and lighter than its predecessor. Its breakthrough design features a large, color touch-screen display with intuitive, vibrant icons that guide the user through the screening process. As a result, users are able to focus on the infant and less on the technology. There are detailed test and results screens, as well as on-screen help menu. Because of its intuitive nature, just minimal training on the device is required to start the screening process.
Entirely Hand-held: No Cart Required!
The new AccuScreen is an all-in-one compact screener: a hand-held device with all the capabilities of a cart-based system, without the cart. Instead of having to transport a computer and printer from room to room, the user simply carries a lightweight hand-held unit to screen patients. At the end of the day, test data can be uploaded to the Acculink database software with one click. “There are so many great features of the new AccuScreen, the best of which are its portability and versatility,” said Karen Morris, Manager, Audiology Services at GN Otometrics North America. “Your computer stays at your desk, where it belongs. The hand-held device is small and light-weight -- making it easy to carry throughout your day. Transferring data between the device and your computer is simple and seamless, with the MADSEN docking station and the Acculink software. We’re giving our customers what they asked for -- an innovative newborn hearing screening device that makes their job easier.”
All-in-one OAE/ABR Device
The new AccuScreen is a combined two step OAE/ABR device that meets all program requirements and interfaces with both the HiTrack and OZ-Systems. It includes multiple OAE protocols and allows for ABR screening at 30, 35, 40 and 45dB. The user login option for data security remains in place and the same low maintenance costs apply to the new system. “The new AccuScreen is revolutionary and will dramatically impact the way nurses and healthcare professionals perform newborn hearing screenings,” said David Adlin, manager, hearing screening products at GN Otometrics North America. “We think that hearing professionals will like what they see because, in reality, they helped us design it. The new AccuScreen is a direct result of our commitment to listening to our customers. We asked them what they needed and wanted in an infant hearing screening device to do their job better and that feedback drove our product innovation. The result is the groundbreaking new AccuScreen.”
About GN Otometrics North America
Copenhagen-based GN Otometrics is the world's leading manufacturer of hearing and balance instruments and software. The company has solutions ranging from infant-screening applications and audiologic diagnostics, to balance testing and hearing-instrument fitting. Visit https://www.otometrics.com/ or the Otometrics Expo Page on AudiologyOnline for more information.
*See www.otometrics.com/accuscreen-us for more information.

