Bethlehem, Pa. Oct. 20, 2010 - Results of the latest study by Neuromonics, Inc., on its Neuromonics tinnitus treatment (NTT) show that treatment response occurs within two months, and improves at all time periods over the first 12 months.The Customized Acoustic Stimulation for Long-Term Medical Benefit (CALM) study is an interim, multi-site, U.S. study that is evaluating patients with chronic disabling tinnitus, a widespread condition characterized by ringing in the ears.
The CALM study's primary objective is to demonstrate clinically significant long-term reductions in tinnitus disturbance and quality-of-life improvements up to 36 months post-treatment with the NTT - an FDA-cleared treatment that addresses the neurological processes of tinnitus by promoting habituation, or desensitization to the patients' tinnitus perception, through spectrally modified music, customized for each patient.
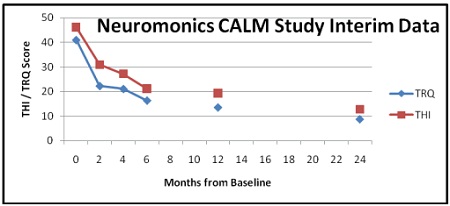
The CALM study used measures of tinnitus disturbance including the Tinnitus Handicap Inventory (THI), a standard clinical measure evaluating the psycho-social consequences of tinnitus;and the Tinnitus Reaction Questionnaire (TRQ);a self-report questionnaire that evaluates tinnitus distress.
Interim results with 51 patients show clinically and statistically significant improvements from the NTT. THI dropped nearly 59 percent, and TRQ fell by 68 percent over 12 months. According to Jack Wazen, M.D., director of ear research with the Silverstein Institute, and medical principal investigator for the CALM study, a reduction of at least 40 percent in the TRQ scores, or 20 points in the THI scores, is considered clinically important.
"These results confirm previous findings, showing a persistence of benefit," says Rick Giancola, CEO of Neuromonics. "By targeting the neurological root causes of tinnitus, Neuromonics helps patients manage and treat their tinnitus and regain control over their lives."
The CALM study is one of 14 studies on NTT that have been completed or are in progress;10 of the studies are independent. Throughout its work, the company remains committed to collaborating with the leading U.S. medical centers, academic hospitals and private practices to demonstrate the efficacy of the NTT, Giancola says. Prestigious academic institutions and private practices participating in the CALM study, initiated in 2007, include the Cleveland Clinic, Silverstein Institute, House Ear Clinic, Michigan Ear Institute, North Shore Audio-Vestibular Lab, Shohet Ear Associates, The Polyclinic, Doctors' Hearing and Balance Centers, ENT Associates of South Florida, and Ear Institute of Chicago.
Neuromonics Tinnitus Treatment (NTT)
Neuromonics' non-invasive device, customized to each patient's individual audiological profile, delivers a neural stimulus that targets the brain's auditory pathways. Clinically administered and monitored, the treatment is a therapy delivered through a compact, lightweight device. Targeting the neurological processes of tinnitus - specifically its audiological, attention-based and emotional aspects - the Neuromonics tinnitus treatment (NTT) typically occurs over an approximately six-month period, with daily use recommended for two or more hours per day.
The NTT is believed to aid in neuroplasticity, the process of neuronal change. The treatment delivers a customized neural stimulus that targets the brain's auditory pathways, and is believed to result in neuroplastic changes that dramatically mitigate patient's disturbance from their tinnitus.
The NTT provides significant long-term reduction of tinnitus disturbance. Research published in the April 2007 issue of Ear & Hearing demonstrates that the treatment yields clinically significant reduction in tinnitus disturbance for more than 90 percent of suitable patients in a formal clinical trial setting.
Neuromonics, Inc.
Neuromonics, Inc., manufactures and distributes the only FDA-cleared, patented and clinically proven medical device designed for long-term significant relief of tinnitus. With research and development beginning in the early 1990s, the Neuromonics tinnitus treatment has helped thousands of tinnitus sufferers improve their quality of life and overcome the daily life challenges associated with tinnitus. Neuromonics is based in Bethlehem, Pa.
For more information about Neuromonics visit www.neuromonics.com.
