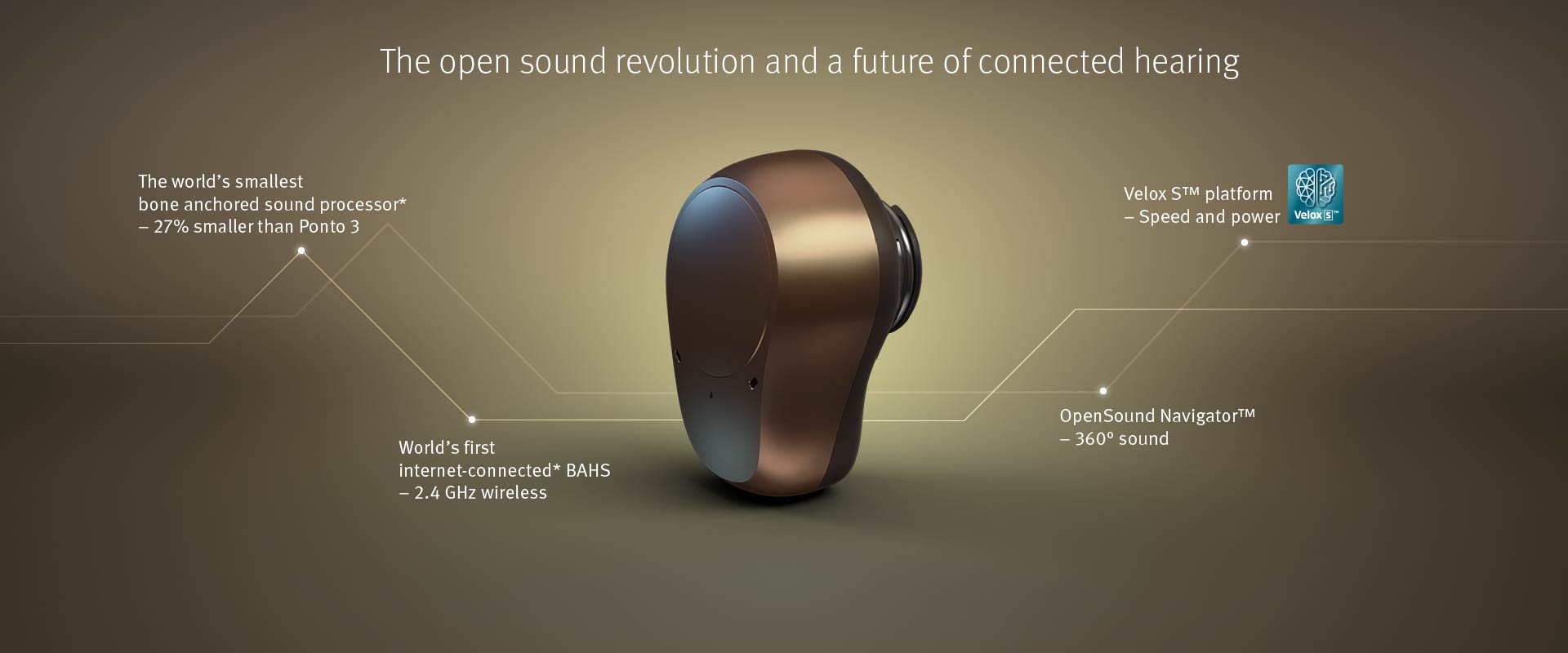
Groundbreaking Sound Processor Is The Smallest Bone Anchored Device On The Market
SOMERSET, NJ May 13 – Oticon Medical announced today that it has obtained 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market the new Ponto 4, a small and high performing sound processor that marks a significant breakthrough in bone anchored hearing care. Ponto 4 will officially launch in the US in early summer 2019.
“With Ponto 4, Oticon Medical enters a new era by bringing the largest improvements for bone anchored users in many years,” says Jes Olsen, President of Oticon Medical. “The introduction of Ponto 4 and the Velox S™ platform signals a paradigm shift in bone anchored hearing. Many users around the world have already had their lives changed by the groundbreaking technology in Oticon Opn™ and Oticon Opn S™ hearing aids. Now, we combine Oticon Medical’s know-how in bone conduction hearing with the newest state-of-the-art technology from one of the world's leading hearing healthcare groups.”
Fast, efficient management of multiple sound sources
Built on the unique Velox S™ platform, Ponto 4 delivers fast and precise sound processing for an open soundscape and noise-optimized listening experience. The groundbreaking OpenSound Navigator™ analyzes the sound environment more than 100 times per second to balance the sound sources and attenuate noise even between words. Users enjoy an open and balanced sound experience.
“With Ponto 4, we are moving away from using directionality as we know it. The OpenSound Navigator handles multiple speech and noise sources in a new, fast and efficient way. It balances the environment and targets noise to a much higher degree that we have been able to before. We feel proud and confident that thiswill make a huge difference in the ability of bone anchored users to follow conversations and keep up with the dynamics of life,” says Olsen.
Small size and wireless connections
The small and appealing Ponto 4 is the smallest bone anchored device on the market, 27% smaller than Ponto 3. The all-new design is built on the hallmark of Ponto reliability and durability to stand up to everyday life.
Ponto 4 will also bring world-first advancements in wireless capabilities to the bone anchored field. Through a unique Oticon cloud solution, the web-based service IFTTT (If This Then That) and the Oticon ON App are used to unlock a world of potential for connected device communication. Users can programme connections between their sound processor to a range of devices and services, for example ”smart” doorbells, smoke detectors and baby alarms. "The Internet of Things brings endless opportunities for Ponto 4 users,” says Olsen. ”What we see now is just the starting point of a future of connected hearing care. The launch of Ponto 4 means bone anchored users can be part of this journey.”
For more information on Oticon Medical and the new Ponto 4 sound processor, visit www.OticonMedical.com.