Some nerve cells in the inner ear can signal tissue damage in a way similar to pain-sensing nerve cells in the body, according to new research from Johns Hopkins. If the finding, discovered in rats, is confirmed in humans, it may lead to new insights into hyperacusis, an increased sensitivity to loud noises that can lead to severe and long-lasting ear pain.
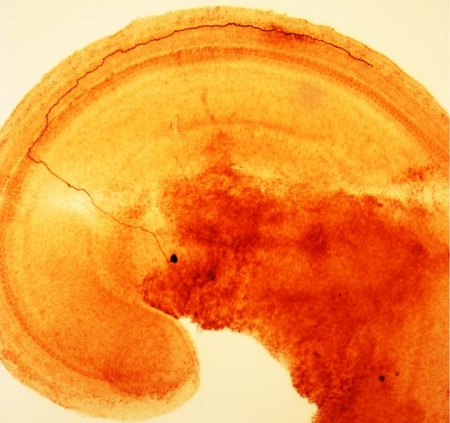
A segment of the inner ear of a rat, showing a type II afferent nerve cell (brown). It begins with a cell body (dark spot, off center) in the spiral ganglion of the brain, crosses the tunnel of Corti and then makes a sharp turn to connect with many outer hair cells. Credit: Fuchs Lab, Johns Hopkins Medicine.
"We are still a long way from being able to treat hyperacusis," says Paul Fuchs, Ph.D., professor of otolaryngology-head and neck surgery, neuroscience and biomedical engineering at the Johns Hopkins University School of Medicine, "but our results suggest that cells called type II afferent neurons are similar to pain-sensing neurons in the rest of the body, so lessons about interventions elsewhere could apply to the ear, too."
A summary of the research will be published online in the journal Proceedings of the National Academy of Sciences during the week of Nov. 9.
The new discovery came as a result of interest in why this small subset of afferent nerve cells -- nerves that take information from the inner ear to the brain -- are quite insensitive to sound. "If they aren't very good at relaying sounds, what are they doing?" says Fuchs.
Fuchs and his team knew that these type II afferents connect to specialized sensory cells in the ear of mammals. These so-called outer hair cells amplify the sound waves that enter the inner ear, giving mammals very sensitive hearing over a wide range of frequencies. But, according to Fuchs, this specialization comes at a cost.
"Outer hair cells are the canaries in the coal mine for the inner ear, in that they're the first cells to die due to loud noise, age or other factors," says Fuchs. "Since they can't regenerate, their death leads to permanent hearing loss." So one possible role for type II afferents, he adds, would be to warn the brain of impending damage to outer hair cells.
It was known that nearby supporting cells respond to outer hair cell damage by increasing their inner calcium levels and releasing the chemical messenger ATP. Fuchs' team knew that type II afferent neurons can respond to ATP, so they damaged outer hair cells while monitoring type II neurons in surgically removed inner ear tissue. Indeed, outer hair cell rupture caused robust excitation of type II neurons.
Fuchs says that the ATP released by the supporting cells is probably what gets the neurons to fire, and the supporting cells might release ATP in response to ATP that leaks out of the ruptured outer hair cells. But he noted that "outer hair cells don't have to rupture to release ATP. Progressive damage caused by loud noises or other stress is enough to increase ATP levels in the fluid of the inner ear."
Over evolutionary time, such a mechanism could have evolved to help mammals avoid further damage to their hearing. Such effects might depend on heightened sensitivity of the type II neurons after trauma, akin to the heightened sensitivity of pain-sensing nerves in damaged skin. Hypersensitivity to loud sound (hyperacusis) is a paradoxical consequence of hearing loss in many people. Everyday noises such as slamming doors, clanking dishes and barking dogs can become irritating and even painful.
The good news, Fuchs says, is that the analogies with pain elsewhere in the body provide guidance for future studies. For example, a compound that suppresses pain-sensing nerve cells elsewhere, also prevented type II afferent neurons from firing in response to outer hair cell death. At present, Fuchs cautions, this is a restricted experimental result. But, it provides a "proof of concept" for treating pain associated with inner ear damage. And the Fuchs laboratory plans to explore this question in their ongoing research.
Other authors of the report include Chang Liu and Elisabeth Glowatzki of the Johns Hopkins University School of Medicine.
This work was supported by grants from the National Institute on Deafness and Other Communication Disorders (R01 DC011741, P30 DC005211).
Source: https://www.eurekalert.org/pub_releases/2015-11/jhm-ncw110915.php

