Researchers have identified mifepristone, an FDA-approved drug for chemical abortion, as a promising candidate for the treatment of vestibular schwannoma.
MASSACHUSETTS EYE AND EAR INFIRMARY - Boston, Mass. --Massachusetts Eye and Ear researchers have shown that mifepristone, a drug currently FDA-approved for chemical abortion, prevents the growth of vestibular schwannoma (also known as acoustic neuroma) cells. This sometimes-lethal intracranial tumor typically causes hearing loss and tinnitus. The findings, published online today in Scientific Reports, suggest that mifepristone is a promising drug candidate to be repositioned for the treatment of these tumors.
"Currently, there are no FDA-approved drugs for vestibular schwannomas or the associated hearing loss," said Konstantina Stankovic, M.D., Ph.D., an ear and skull base surgeon and auditory neuroscientist at Mass. Eye and Ear and Harvard Medical School who led the study. "Therefore, there is an unmet medical need to discover drugs with minimal adverse effects that would treat this tumor and reduce or obviate the need for surgery and radiation."
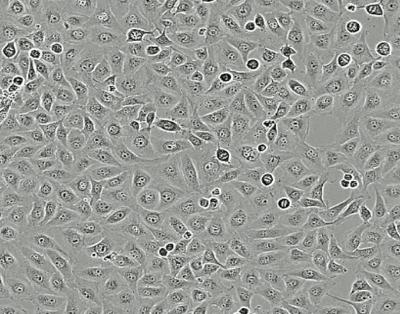
Vestibular schwannoma cells from an untreated tumor. Image courtesy of Massachusetts Eye and Ear.
Although histologically non-malignant, vestibular schwannomas are dangerous due to their location. Arising from the Schwann cells of the vestibular (balance) nerve, these tumors can grow to the point of damaging nearby structures -- and can lead to death by compressing the brainstem. By compressing nerves in the internal auditory canal, the tumors can cause dizziness and facial nerve paralysis, in addition to hearing loss.
Though vestibular schwannomas affecting both sides are the hallmark of neurofibromatosis type 2 (NF2), a genetic disorder causing tumors to grow at multiple sites throughout a person's life, vestibular schwannomas may also occur sporadically, and on one side only.
Currently, patients with symptomatic or growing vestibular schwannomas can undergo surgical resection (through craniotomy) or radiation therapy; however, both of these procedures come with significant risks.
In the Scientific Reports study, researchers first performed the largest meta-analysis of the vestibular schwannoma transcriptome by studying 80 tumors from patients with sporadic and NF2-associated vestibular schwannomas. Then, through a survey of more than 1,100 drug candidates (all FDA-approved), they identified a short list of drugs likely to convert the abnormal transcriptional signature of a tumor to a more normal one. They then tested eight of the most promising candidate drugs against cells grown from other human vestibular schwannomas. Their experiments showed that one drug in particular, mifepristone, was most effective. Treatment of vestibular schwannoma cells with mifepristone reduced their proliferation rate by 80 percent.
"Our investigation represents the first application of algorithm-based repositioning of FDA-approved drugs for this tumor class," said first author Jessica Sagers, a PhD student at Harvard Medical School.
Mifepristone is an attractive candidate for repurposing, as it is relatively safe, well studied and carries minimal adverse effects. FDA-approved in 2000, mifepristone is most often used together with another medication, misoprostol, to end an early pregnancy. Adverse effects for mifepristone include mild fatigue, hot flashes, nausea and rash. Long-term use of mifepristone has been studied in clinical trials for other tumors, with minimal adverse effects reported after years of usage.
Based on the findings described in Scientific Reports, the study authors are cautiously optimistic about the therapeutic potential of mifepristone for patients with vestibular schwannomas, either from NF2 or those arising sporadically. Dr. Stankovic hopes to begin a phase II clinical trial at Mass. Eye and Ear soon to determine efficacy of the drug for this indication.
"We are optimistic about the potential to repurpose this relatively safe drug for patients desperately in need of better solutions," she said.
###
In addition to Stankovic and Sagers, authors on the Scientific Reports paper include co-authors Adam Brown, Isaac Kohane and Chirag Patel of Harvard Medical School, Sasa Vasilijic, Rebecca Lewis, Mehmet Sahin, Lukas Landegger and Bradley Welling of Mass. Eye and Ear/Harvard Medical School, and Roy H. Perlis of Mass General/Harvard Medical School.
This research was supported by National Institute of Deafness and Other Communications Disorders grants R01DC015824 and T32DC00038, National Human Genome Research Institute grants R00ES023504, R21ES025052, and T32HG002295 and Department of Defense grant W81XWH-15-1-0472. Additional support included research grants from the Bertarelli Foundation, the Nancy Sayles Day Foundation, the Zwanziger Foundation, the Barnes Foundation, the Lauer Tinnitus Research Center and the Kutchin Foundation.
About Massachusetts Eye and Ear
Massachusetts Eye and Ear, founded in 1824, is an international center for treatment and research and a teaching hospital of Harvard Medical School. Specializing in ophthalmology (eye care) and otolaryngology-head and neck surgery (ear, nose and throat care), Mass. Eye and Ear clinicians provide care ranging from the routine to the very complex. Also home to the world's largest community of hearing and vision researchers, Mass. Eye and Ear has pioneered new treatments for blindness, deafness and diseases of the head and neck. Our scientists are driven by a mission to discover the basic biology underlying these conditions and to develop new treatments and cures. In the 2017-2018 "Best Hospitals Survey," U.S. News & World Report ranked Mass. Eye and Ear #2 in the nation for ear, nose and throat care and #4 for eye care. For more information about life-changing care and research at Mass. Eye and Ear, please visit our blog, Focus, and follow us on Twitter and Facebook.
Source: https://www.eurekalert.org/pub_releases/2018-04/meae-mmh040318.php

