Researchers deliver CRISPR-Cas9 directly into the inner ear hair cells of mice, preventing hearing loss in animal model of genetic progressive deafness.
MASSACHUSETTS EYE AND EAR INFIRMARY -
WHAT THEY FOUND
- A CRISPR-Cas9 gene editing complex can be delivered directly into hair cells, the sound-sensing cells of the inner ear, in mice to disrupt a mutated gene that causes progressive hearing loss in mice and humans.
- With the mutated gene disabled, the inner ear hair cells survive, and mice otherwise genetically destined to become deaf retain a portion of their hearing.
- At four weeks, untreated mice were unresponsive to sound below an average of 80 decibels, while treated mice responded to sound at approximately 65 decibels
- At eight weeks, treated mice also retained their instinctive physical "startle" response to sudden loud sound, while the untreated mice did not respond.
WHY IT MATTERS
- Hearing loss is the most common form of sensory loss in humans, and almost half of cases have an underlying genetic component.
- The study is the first to demonstrate a genome-editing protein complex introduced into an animal model of genetic hearing loss with therapeutic benefit.
- Direct local delivery of Cas9 protein:guide RNA:lipid complex allows exquisite DNA specificity, needed to selectively disrupt the pathogenic copy of the gene without disrupting the normal copy of the gene. In contrast, delivery of DNA encoding the Cas9 and guide RNA results in more modest DNA specificity and greater potential side effects.
- The approach of delivering the Cas9 gene-editing protein complex directly into the relevant tissue could be applied to treat other disorders amenable to gene editing therapies.
Cambridge, Boston, MA (Dec. 20, 2017) -- Hearing loss is the most common form of sensory loss in humans, and almost half of cases have an underlying genetic cause. Reporting today in Nature, a team led by researchers from the Broad Institute of MIT and Harvard, Massachusetts Eye and Ear, Harvard University, and Howard Hughes Medical Institute (HHMI) have developed a CRISPR-Cas9 genome-editing therapy to prevent hearing loss in a mouse model of human genetic progressive deafness.
The therapy delivers a CRISPR-Cas9 gene-editing protein complex directly into the sound-sensing cells of the inner ear (known as "hair cells") to disrupt a mutation that would otherwise cause the cells to die. The work represents the first time that a genome-editing protein has been ferried directly into the relevant cells to halt progression of genetic hearing loss. Delivering the Cas9 protein itself locally, instead of DNA elements that the cell can use to build Cas9, improved the DNA specificity and potential safety of the treatment.
"We set out to develop a genome-editing strategy to try to address this genetic hearing loss by disrupting the underlying genetic variant," said co-senior author David Liu, the Richard Merkin Professor, director of the Merkin Institute of Transformative Technologies in Healthcare, and core institute member at the Broad Institute, professor of chemistry and chemical biology at Harvard University, and HHMI investigator. "A lot of additional work is needed before this strategy might inform the development of a therapy for humans, but at this stage, we're delighted and excited that the treatment preserved some hearing in the animal model."
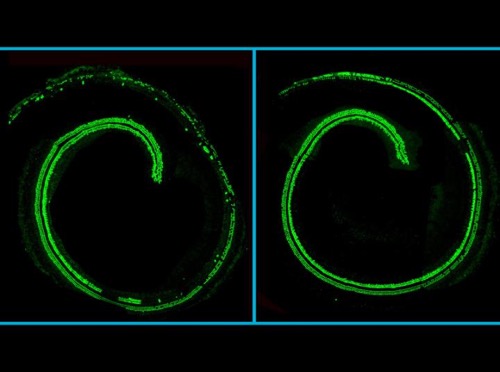
Confocal microscopy images of mouse cochlea; hair cells in green. (Left) An untreated cochlea from a mouse with the Tmc1 mutation, displaying hair cell loss. (Right) The opposite, treated, cochlea from the same mouse, displaying hair cell preservation. Source: Gao et al./Nature 2017.
Hair cells are the specialized inner-ear cells that turn the mechanical vibrations of sound waves into electrical signals that the brain can interpret. One root cause of genetic hearing loss is a single-letter mutation in a gene called TMC1 that causes hair cells to produce a malformed, toxic protein, one which builds up and kills the cell. Humans (and mice) with this mutation suffer progressive hearing loss during youth, and eventually become profoundly deaf.
Because the mutated TMC1 gene only differs from its normal counterpart by a single letter of DNA, Cas9 must target the mutated gene with laser precision. Otherwise, the Cas9 protein would easily cut and disable the functional copy of the gene instead.
With an eye towards improving the editing precision, the team built on prior work they had published in 2014. When it comes to using CRISPR-Cas9 for gene editing, researchers typically insert the DNA encoding the Cas9 complex into a cell, and let the cell use its own machinery to produce the gene-editing arsenal. But Liu and colleagues previously demonstrated that if the Cas9 gene-editing complex itself was delivered directly into a cell, packaged inside an envelope of lipids, the editing was much more precise. Cas9 itself degrades quickly, and generally cuts its exact target first before moving on to edit similar "off-target" DNA strands -- so, when delivered as a protein complex, it usually breaks down before it has a chance to make those mistakes.
"The strategy we used was particularly efficient in targeting dominant genetic hearing loss," said co-senior author Zheng-Yi Chen, associate professor at Massachusetts Eye and Ear. "In humans, dominant hearing loss generally manifests as late-onset and progressive, therefore providing us with a precious time window for intervention. The therapeutic effect through local inner ear delivery also presents a major advantage in reducing potential risks."
The researchers tested the method in a mouse model of progressive hearing loss with a mutated Tmc1 gene. Left untreated, the mice experience hearing loss by four weeks of age and profound deafness at eight. The team injected the gene-editing mix into the cochlea of newborn mice genetically destined for profound hearing loss.
The treated mice maintained a substantial amount of their hearing compared to the untreated mice. At four weeks, the untreated mice had a measurable response in their brainstem to sound starting at roughly 80 decibels, the volume of a garbage disposal or a loud radio. But the treated mice responded to sound starting around 65 decibels -- approximately the same volume as a typical spoken conversation.
Physiological measurements showed that the hair cells survived at a higher rate in the treated cochlea; genetic sequencing showed that among the edited cells, the mutated copy of Tmc1 had successfully been disrupted 94 percent of the time, and the wild-type allele had only been hit 6 percent of the time. At eight weeks, treated mice also retained their instinctive physical "startle" response to sudden loud sound, while the untreated mice did not respond.
"This is an exciting study that demonstrates the feasibility of a DNA-free, virus-free genome editing strategy for a type of autosomal dominant hearing loss characterized by progressive hair cell loss," said Tina Stankovic, an associate professor at Mass. Eye and Ear who was not involved with the study. "Augmenting the toolbox to treat genetic deafness is of major significance."
The team plans to develop the therapy in larger animal models of genetic progressive hearing loss. "These results inform the potential development of a treatment for a subtype of genetic hearing loss, but making sure the method is safe and effective is critically important before we propose moving closer to human trials," said Liu. "We also recognize the importance and remain mindful of cultural considerations within the Deaf community as this work moves forward."
###
This work was supported by DARPA (HR0011-17-2-0049), the NIH (R01 EB022376, R35 GM118062, R01 DC006908, P30 DC05209, R01 DC00138, and R01 DC013521), the David-Shulsky Foundation, a Frederick and Ines Yeatts Hair Cell Regeneration grant, the Bertarelli Foundation, the Broad Institute, and HHMI.
Paper cited:
Gao X and Tao Y, et al. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature. Online December 20, 2017. DOI: 10.1038/nature25164
About the Broad Institute of MIT and Harvard
Broad Institute of MIT and Harvard was launched in 2004 to empower this generation of creative scientists to transform medicine. The Broad Institute seeks to describe all the molecular components of life and their connections; discover the molecular basis of major human diseases; develop effective new approaches to diagnostics and therapeutics; and disseminate discoveries, tools, methods, and data openly to the entire scientific community.
Founded by MIT, Harvard, Harvard-affiliated hospitals, and the visionary Los Angeles philanthropists Eli and Edythe L. Broad, the Broad Institute includes faculty, professional staff, and students from throughout the MIT and Harvard biomedical research communities and beyond, with collaborations spanning over a hundred private and public institutions in more than 40 countries worldwide. For further information about the Broad Institute, go to https://www.
About Massachusetts Eye and Ear
Mass. Eye and Ear clinicians and scientists are driven by a mission to find cures for blindness, deafness and diseases of the head and neck. Now united with Schepens Eye Research Institute, Mass. Eye and Ear is the world's largest vision and hearing research center, developing new treatments and cures through discovery and innovation. Mass. Eye and Ear is a Harvard Medical School teaching hospital and trains future medical leaders in ophthalmology and otolaryngology, through residency as well as clinical and research fellowships. Internationally acclaimed since its founding in 1824, Mass. Eye and Ear employs full-time, board-certified physicians who offer high-quality and affordable specialty care that ranges from the routine to the very complex. In the 2017-2018 "Best Hospitals Survey," U.S. News & World Report ranked Mass. Eye and Ear #2 in the nation ear, nose and throat care and #4 for eye care. For more information about life-changing care and research, or to learn how you can help, please visit https://www.
Source: https://www.eurekalert.org/pub_releases/2017-12/meae-ctp121817.php

