Auditory neuroscientists discover bottleneck of information flow in the ear and pave the way for gene therapy of deafness. Publication in ‘EMBO Journal’.
Disabling hearing impairment (HI) affects 360 million people worldwide, and prevalence increases with age. So far, no causal treatment is available for its most common form, sensorineural HI. Göttingen and Berlin scientists have achieved a major advance in our understanding of hearing as well as an important step towards developing gene therapy of deafness. Their study showed that the endocytic adaptor protein 2µ is required for hearing by fueling vesicle reloading of the release site for indefatigable synaptic transmission. Without AP-2, which inter-acts with the deafness protein otoferlin, a kind of traffic jam occurs at the release sites, suggesting that AP-2 and Otoferlin teamwork in clearing exocytosed material from the release site. Using virus-mediated transfer of the intact AP-2µ DNA into sensory inner hair cells, the scientist could restore normal synaptic function and hearing.
The research was performed by scientists of several research institutions at the Göttingen Campus within the collaborative research center 889 (CRC) “Cellular Mechanisms of Sensory Processing” and by scientists of the Berlin Leibniz-Institute for Molecular Pharmacology. The work was published in the EMBO Journal.
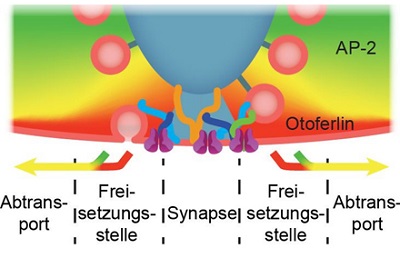
Figure 1: Active zone clearance; After fusion of synaptic vesicles (red spheres) with the plasma membrane, interaction of AP-2 and otoferlin along opposing protein gradients is hypothesized to enable efficient clearance of exocytosed proteolipid from the release site. Abb.: Jung et al., EMBO J 2015.
Specialized contact points between sensory hair cells and auditory nerve cells, the so-called “ribbon” synapses, convert acoustic information into a neural code in the inner ear. The rate of synaptic transmission amounts to amazing hundreds per second, requiring a highly efficient and indefatigable recruitment, fusion and recy-cling of synaptic vesicles at the active zones of transmitter release. This extraordi-nary performance involves Otoferlin that is defective in sensorineural deafness. However, the underlying interactions of Otoferlin with other proteins of the synapse are not well understood.
Ultrafast replenishment of release sites requires their efficient clearance after fusion
What limits the rate of transmission at the hair cell synapse? How does Otoferlin enable indefatigable transmitter release? Each of the sub-micrometer-sized active zones likely can relase up to approximately 1000 transmitter-filled synaptic vesicles per second. Such high traffic volume causes loads of vesicular proteins and lipid to strand in the membrane of the active zone. It seems that the synapse employs dedicated mechanisms to clear this stranded material from the fusion zone to the clearance zone from which it will be recycled by endocytosis (re-uptake into the cell). Efficient “active zone clearance” is likely important for ultrafast reloading of the release sites (Figure 1). How this works, so far, was unclear.
Using transgenic mouse lines generated in Berlin and Göttingen, scientists now discovered that the adaptor protein AP-2µ, a crucial component of the endocytic machinery, plays an important role in active zone clearance. Mice are deaf when their hair cells lack AP-2µ. Deafness results from slowed vesicle reloading at the release sites of the active zones, as demonstrated by a multidisciplinary approach. Dr. Carolin Wichmann, group leader at the Institute for Auditory Neuroscience of the University Medical Center Göttingen and one of the first authors, says: „We were surprised, that transmitter release slowed down already at 20 thousandth of a second of stimulation. Previously, AP-2 was reported to primarily work in the slower process of vesicle recycling.“ In order to understand the underlying molecular mechanism, the scientists also studied the interactions of AP-2 and found binding to Otoferlin, a molecule that is defect in human deafness, which also supports vesicle replenishment at the active zone. Dr. Tanja Maritzen, group leader at the Leibniz-Institute for Molecular Pharmacology, Berlin, and a first author says: „We found AP-2 and Otoferlin to interact at least via two binding sites. Moreover, our experiments revealed that AP-2µ is critical for maintaining normal levels of Otoferlin.“
But how can the interaction of AP-2 and Otoferlin speed vesicle replenishment at the active zone? Dr. Andreas Neef, group leader at the Göttingen Bernstein Center for Computational Neuroscience and MPI for Dynamics and Self-Organisation, a corresponding author states: „Based on combining systems physiological recordings of transmitter release at single active zone, a unique opportunity at these synapses, with mathematical modeling, we postulate that AP-2 speeds clearance of active zones via binding to Otoferlin.“ Thereby, the scientists believe, exocytosed material can be removed more quickly enabling new vesicles to come in and prepare for the next round of transmitter release. Lack of AP-2 or Otoferlin would then cause a “traffic jam” and impair sound encoding: deafness results.
This study is one of the first world-wide that demonstrates the feasibility of using non-pathogenic virus for gene-replacement therapy in animal models. Dr. SangYong Jung, scientist at the Institute for Auditory Neuroscience of the University Medical Center Göttingen and one of the first authors says: „When injecting adeno-associated virus carrying the DNA for AP-2µ into the cochlea of these deaf mice, we could restore the function of the hair cell synapses and hearing.“ The leaders of the study Dr. Volker Haucke (Director of the Leibniz-Institute for Molecular Pharmacology and Professor at the Free University of Berlin) and Dr. Tobias Moser (Director of Institute for Auditory Neuroscience at the University Medical Center Göttingen and Max-Planck-Fellow at the MPIs for Biophysical Chemistry and Experimental Medicine) agree that this study offers an important advance of our understanding of the function of AP-2 in synaptic transmission and, at the same time, paves the way for future gene therapy of deafness. Haucke: “The high-throughput performance of the auditory system allowed us to better understand the function of AP-2 at the active zone. AP-2 and Otoferlin seem to teamwork in “active zone clearance” in order to realize the spectacular rates of transmission required for hearing”. Moser adds: „While so far we don’t know of a human deafness caused by mutations in AP-2, our study raises hopes that virus-mediated gene therapy can be translated into the clinic in the near future. The near normal hearing after gene therapy of one ear and the lack of virus spread (e.g. to the other ear) indicate that early intervention could restore hearing in select genetic deafness.”
Publication
Sangyong Jung#, Tanja Maritzen#, Carolin Wichmann#, Zhizi Jing, Andreas Neef§, Natalia H. Revelo, Hanan Al-Moyed, Sandra Meese, Sonja M. Wojcik, Iliana Panou, Haydar Bulut, Peter Schu, Ralf Ficner, Ellen Reisinger, Silvio O. Rizzoli, Jakob Neef, Nicola Strenzke, Volker Haucke§ and Tobias Moser§ (2015). Disruption of adaptor protein 2μ (AP-2μ) in cochlear hair cells impairs vesicle reloading of synaptic release sites and hearing. EMBO Journal, DOI: 10.15252/embj.201591885, online: Oct 7th, 2015
# gleichwertiger Beitrag zum Artikel
§ korrespondierender Autor
Source: https://www.fmp-berlin.info

