Possible Pathomechanisms Underlying a Spontaneous Reversal of Positional Nystagmus in PSC - BPPV
•Pathomechanisms potentially explaining RPN, including peripheral short-term adaptations of the vestibulo-ocular reflex (VOR) or the hypothesis of simultaneous lithiasis in both ampullary and non-ampullary canal arms.
•The pivotal role of the video head impulse test (vHIT) in diagnosing and guiding physical therapy for complex BPPV cases associated with RPN.
•The therapeutic challenges in managing BPPV with RPN, particularly the need for repeated canalith repositioning maneuvers.
This interview offers a comprehensive overview of RPN, combining theoretical explanations and practical insights, while emphasizing the importance of advanced diagnostic tools in effectively managing these complex conditions.
Introduction
Benign paroxysmal positional vertigo (BPPV) represents a common disorder in which otoconia detach from the utricular macula and settle one or more sites of a semicircular canal (SC). This condition results in vertigo spells triggered by head position changes due to an abnormal activation of the ampullary afferents of the involved SC. Different conditions can be set up depending on how and where otoconia are disposed within the canal, as they:
- can float within the canal in canalolithiasis
- might adhere to the cupula in cupulolithiasis
- remain entrapped within stenotic tract of the membranous SC, plugging the canal lumen, in canalith jam.
The posterior SC (PSC) represents the most commonly involved site due to its undermost position. The diagnosis usually relies on the interpretation of the eye movements due to head position changes with respect to the gravity. Nevertheless, the role of the video-head impulse test (vHIT) in the detection of the affected SC in particular challenging cases has emerged in recent years. In fact, recent studies have demonstrated how the vestibulo-ocular reflex (VOR) of the affected SC at the vHIT might be impaired in some cases of apogeotropic PSC-BPPV (where otoconia are thought to settle the non-ampullary arm of the canal) and in rare cases of anterior SC (ASC) BPPV (where otoconia are thought to settle a stenotic tract of the ampullary arm of the canal). In both these cases, downbeat/torsional nystagmus elicited in the provoking positions either due to an inhibition of the PSC afferents or due to an excitation of the ASC afferents, respectively, in particular when persistent, might be due to a positional/incomplete canalith jam acting as a low-pass filter for the endolymphatic flows, preventing high-frequency responses of the SC while allowing low frequency stimuli (i.e. otoconial shifts).
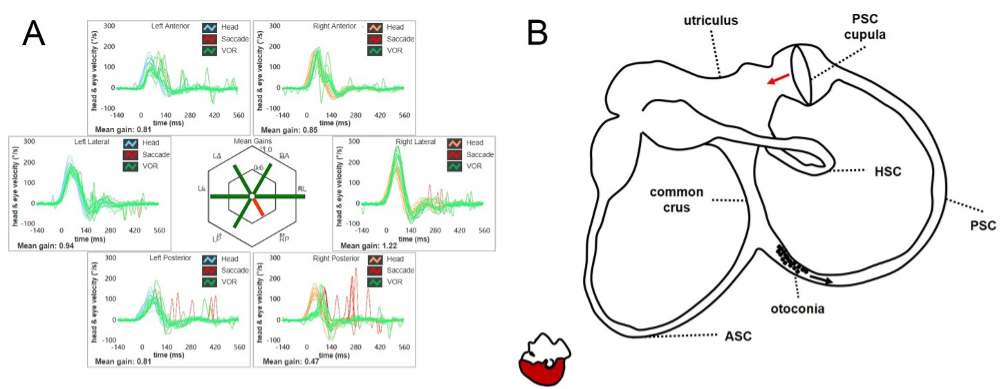
Figure 1. Apogeotropic PSC-BPPV leading to a reduction for the VOR-gain for the affected canal (in this case the right PSC) at the vHIT (A). Otoliths are partially entrapped within a narrow tract of the non-ampullary arm of the canal. The canal is thought to be partially plugged by otoconia (incomplete jam), explaining the high-frequency VOR-gain impairment for the involved canal (B). Once the diagnostic head hanging position is obtained, the gravity vector slowly moves debris toward the ampullary tract of the canal below, leading to a persistent ampullopetal endolymphatic flow as the otoconial clot in this position acts as a canalith jam (positional jam) on the cupula. The persistent cupular bending generates an inhibitory discharge for the PSC afferents, accounting for persistent positional downbeat nystagmus. (From Castellucci A, Malara P, Martellucci S, et al. Front Neurol. 2020).
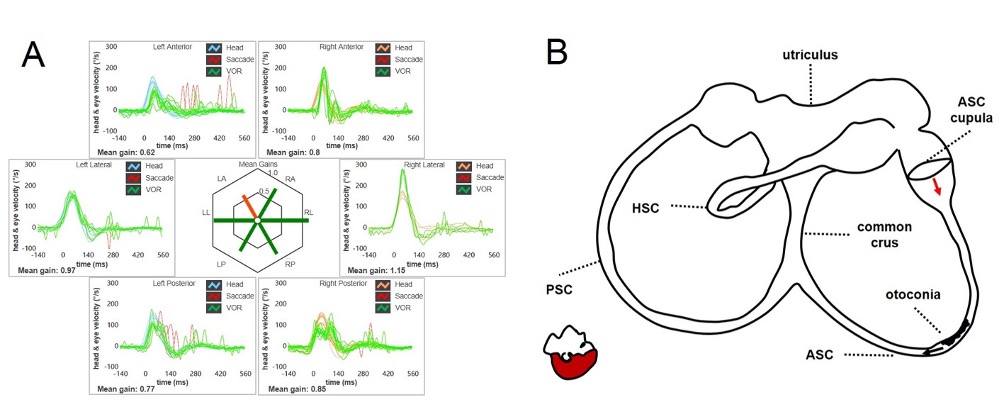
Figure 2. Even ASC-BPPV may result in a selective reduction of the VOR-gain values for the involved canal (in this case the left ASC) at the vHIT (A). Debris are located within a nearly horizontal tract of the canal, where possible narrowing or irregularities could allow them to settle there. Even in this condition, otoconia are thought to partially occlude the canal lumen, only preventing the high-frequency flows as detected at the vHIT (B). At the positioning tests, otoconia slowly shift toward the non-ampullary tract of the canal due to gravity acting as a positional jam. The endolymphatic movement lead the ASC cupula to bend ampullofugally (excitation), accounting for persistent positional downbeat nystagmus. (From Castellucci A, Malara P, Martellucci S, et al. Front Neurol. 2020).
On the other hand, most investigations detected a normal VOR for the affected SC at the vHIT in typical PSC-BPPV. In fact, in this condition, debris are free to float back and forth along the ampullary arm of the canal leading to transient paroxysmal positional nystagmus in the ipsilateral provoking position (Dix Hallpike or Semont position), and they do not alter high-frequency endolymphatic dynamics.
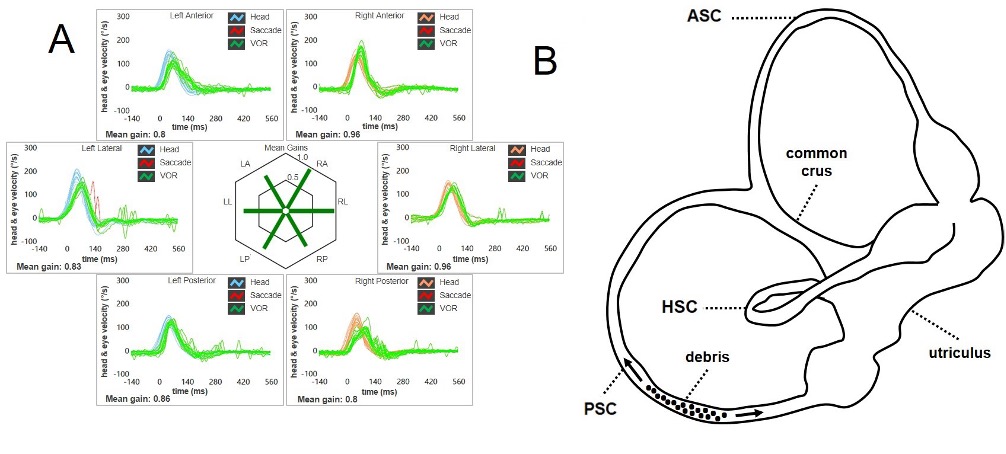
Figure 3. In typical PSC-BPPV, the VOR-gain for the involved canal (in this case the right PSC) is not affected at the vHIT (A). In fact, debris are free to float back and forth along the canal without altering the cupular dynamics in the high-frequency range (B). (From Castellucci A, Malara P, Martellucci S, Delmonte S, Ghidini A. Otol Neurotol. 2021).
AudiologyOnline: What is a spontaneous reversal of positional nystagmus?
Andrea Castellucci: The so-called “Reversing Positional Nystagmus” (RPN) is a nystagmus that spontaneously reverses its direction while the patient remains in the same position. It has been frequently described in lateral SC (LSC) BPPV at the supine head roll test. In fact, it is quite common to observe a long-lasting apogeotropic nystagmus following a strong paroxysmal horizontal geotropic nystagmus while the patients is in the same position. Though it has been more frequently observed at the supine head roll test on the affected side, it might be also detected in the contralateral position. Similarly, in some cases of PSC-BPPV paroxysmal upbeat nystagmus with ipsilateral torsional components in the Dix Hallpike position might be spontaneously replaced by a long-lasting reverse nystagmus (i.e. with opposite direction) aligning with the same canal plane (downbeat with contralesional torsional components). The former is RPN in LSC-BPPV while the last one represents RPN in PSC-BPPV. Most authors agree with the assumption that patients presenting with RPN might need more canalith repositioning maneuvers (CRM) than patients with typical BPPV.
AudiologyOnline: Which are the pathomechanisms that have been traditionally involved in the explanation of a spontaneous reversal of positional nystagmus?
Andrea Castellucci: The pathomechanisms underlying RPN have been controversial for several years and most studies have analyzed the phenomenon in the case of LSC-BPPV. They might be summarized as follows:
- The first-phase geotropic nystagmus in LSC-BPPV would result from an ampullopetal flow while the second-phase apogeotropic nystagmus would originate from ampullofugal flows consistent with cupular deflections due to an endolymphatic reflux resulting in a reversal of the endolymphatic flows.
- The inversion of the direction of nystagmus could occur due to peripheral short-term adaptation mechanisms of the VOR of the involved SC. In line with this assumption, it has been observed that RPN occurred in patients with LSC-BPPV when the slow-phase velocity of the first-phase geotropic nystagmus was higher than 50 deg/s.
- It has also hypothesized that the coexistence of canalolithiasis and cupulolithiasis could explain RPN in LSC-BPPV. Nevertheless, this assumption does not fit RPN in PSC-BPPV as PSC cupulolithiasis should not lead to a reversal downbeat nystagmus, but rather to a persistent upbeat/torsional nystagmus.
- Other Authors have also suggested the role of central short-term adaptation mechanisms in the origin of RPN in LSC-BPPV
AudiologyOnline: How to explain the spontaneous reversal of positional nystagmus in PSC BPPV?
Andrea Castellucci: To date, few cases of RPN involving the PSC have been analyzed.
Even though the role of all the aforementioned mechanisms cannot be excluded, a simultaneous lithiasis involving the ampullary arm and the non-ampullary arm of the PSC might represent the easiest explanation for RPN in PSC-BPPV. Based on the premises summarized in the introduction, it is possible to assume an etiopathogenetic role of an incomplete canalith jam involving the non-ampullary arm of the affected PSC for the cases presenting with PSC-RPN and an associated PSC hypofunction on vHIT.
RPN in PSC-BPPV could be therefore generated as follows:
- a typical canalolithiasis involving the ampullary arm of PSC would generate the first-phase paroxysmal nystagmus (e.g. positional paroxysmal upbeat nystagmus with ipsilateral torsional component) at the Dix Hallpike/Semont test
- an incomplete/positional canalith jam of the non-ampullary arm of the same canal would account for the second-phase persistent nystagmus (e.g. persistent positional downbeat nystagmus with contralesional torsional components).
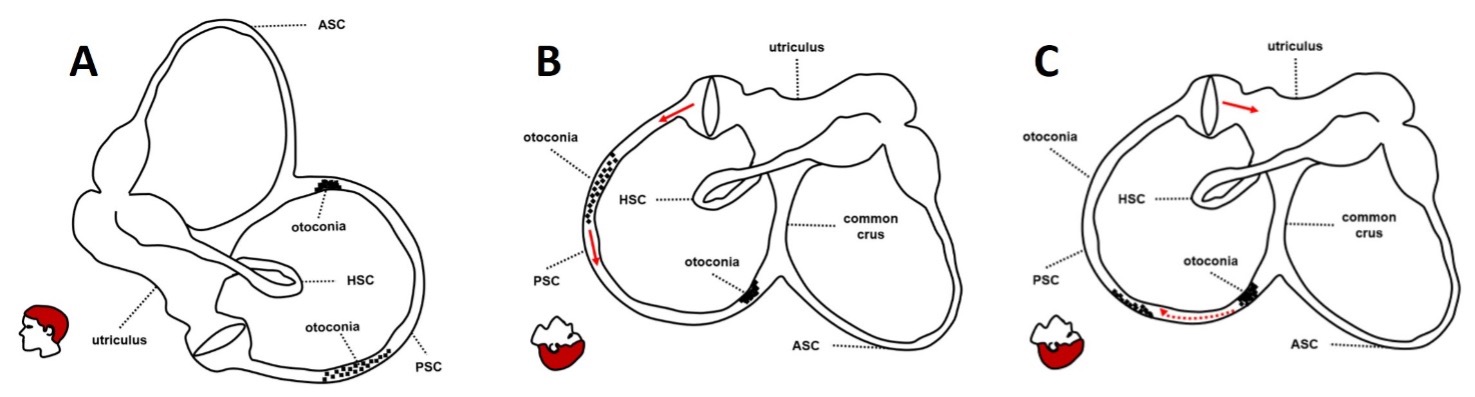
Figure 4. Possible explanation for RPN in PSC-BPPV (in this case the left PSC). Two separate groups of otoconia settle the PSC: free-floating debris in the ampullary arm and an incomplete canalith with debris partially entrapped in the non-ampullary arm (A). In provoking position, free-floating debris in the ampullary arm first move away from the ampulla, resulting in a transient excitation of the PSC afferents and in paroxysmal upbeat/left-torsional nystagmus (B). Then, partially entrapped debris in the non-ampullary arm of the canal move toward the ampulla and block the PSC lumen resulting in an incomplete/positional canalith jam and in persistent positional downbeat nystagmus (C). (From Cocchi C, Castellucci A, Malara P, et al. J Vestib Res (under review)).
According to this theory, the cases of PSC-RPN with associated canal hypofunction a the vHIT could be attributed to a simultaneous ampullary and non-ampullary BPPV of the PSC.
This hypothesis is supported by several observation:
- A positional persistent downbeat nystagmus is often observed even at the positioning test on the opposite side of the affected PSC, similarly to the apogeotropic PSC-BPPV which result in downbeat positional nystagmus on both Dix Hallpike positionings.
- The limited short-term effectiveness of the CRM, as the majority of the cases with RPN requires more than a single treatment session. In fact, the presence of an incomplete canalith jam settling the non-ampullary arm likely prevents free-floating otoliths to reach the utricle from the ampullary arm. This otolith clot likely represents a barrier to the cleaning of the canal with CRM, accounting for the refractory nature of PSC- BPPV presenting with RPN.
- Interestingly, it is also possible to measure an improvement in the VOR-gain values for the affected PSC after the resolution of the BPPV, in accordance with the cases of BPPV involving the vertical canals with persistent positional downbeat nystagmus.
- A higher number of patients with RPN-PSC and PSC hypofunction seem to have a history of previous ipsilateral PSC-BPPV compared to patients with RPN-PSC but no PSC hypofunction. Recurrent BPPV spells might have facilitated the dispersion of detached otoconia in more than a single tract of the canal, predisposing to the development of a simultaneous ampullary and non-ampullary PSC-BPPV
On the other hand, the role of other etiopathogenetic mechanisms cannot be excluded for the cases of PSC-RPN without detectable high-frequency VOR deficit. Based on these observations, it is not possible to exclude the contribution of peripheral or central adaptation mechanisms, in particular for those cases with PSC-RPN, normal VOR-gain values and intense first-phase nystagmus.
Considering the results of the study, the vHIT undoubtedly plays a cardinal role in allowing both a proper diagnosis and an adequate physical therapy for the cases of BPPV with PSC-RPN.
References
- Agus, G., Puxeddu, R., Demontis, G. P., & Puxeddu, P. (1995). Atypical "reversed" paroxysmal positioning nystagmus in benign paroxysmal positional vertigo. Acta Otolaryngologica Supplementum, 520(Pt 1), 143-147.
- R.W. Baloh, K. Jacobson, and V. Honrubia, Horizontal semicircular canal variant of benign positional vertigo, Neurology 43 (1993), 2542–2549.
- Bertholon, P., Bronstein, A. M., Davies, R. A., Rudge, P., & Thilo, K. V. (2002). Positional down beating nystagmus in 50 patients: Cerebellar disorders and possible anterior semicircular canalithiasis. Journal of Neurology, Neurosurgery, and Psychiatry, 72, 366-372.
- Brandt, T. (1990). Positional and positioning vertigo and nystagmus. Journal of the Neurological Sciences, 95, 3-28.
- Büki, B., Mandalà, M., & Nuti, D. (2014). Typical and atypical benign paroxysmal positional vertigo: Literature review and new theoretical considerations. Journal of Vestibular Research, 24, 415-423.
- Califano, L., Capparuccia, P. G., Di Maria, D., Melillo, M. G., & Villari, D. (2003). Treatment of benign paroxysmal positional vertigo of posterior semicircular canal by "Quick Liberatory Rotation Manoeuvre". Acta Otorhinolaryngologica Italica, 23(3), 161-167.
- Califano, L., Salafia, F., Mazzone, S., Melillo, M. G., & Califano, M. (2014). Anterior canal BPPV and apogeotropic posterior canal BPPV: Two rare forms of vertical canalolithiasis. Acta Otorhinolaryngologica Italica, 34, 189-197.
- Califano, L., Iannella, R., Mazzone, S., Salafia, F., & Melillo, M. G. (2021). The video head impulse test in the acute stage of posterior canal benign paroxysmal positional vertigo. Acta Otorhinolaryngologica Italica, 41(1), 69-76.
- Cambi, J., Astore, S., Mandalà, M., Trabalzini, F., & Nuti, D. (2013). Natural course of positional down-beating nystagmus of peripheral origin. Journal of Neurology, 260, 1489-1496.
- Castellucci, A., Malara, P., Brandolini, C., Del Vecchio, V., Giordano, D., Ghidini, A., Ferri, G. G., & Pirodda, A. (2019). Isolated horizontal canal hypofunction differentiating a canalith jam from an acute peripheral vestibular loss. American Journal of Otolaryngology, 40, 319-322.
- Castellucci, A., Malara, P., Delmonte, S., & Ghidini, A. (2020). A possible role of video-head impulse test in detecting canal involvement in benign paroxysmal positional vertigo presenting with positional downbeat nystagmus. Otology & Neurotology, 41, 386-391.
- Castellucci, A., Malara, P., Martellucci, S., Botti, C., Delmonte, S., Quaglieri, S., Rebecchi, E., Armato, E., Ralli, M., Manfrin, M. L., Ghidini, A., & Asprella Libonati, G. (2020). Feasibility of using the video-head impulse test to detect the involved canal in benign paroxysmal positional vertigo presenting with positional downbeat nystagmus. Frontiers in Neurology, 11, 578588.
- Castellucci, A., Malara, P., & Ghidini, A. (2021). Spontaneous downbeat nystagmus in posterior semicircular canal benign paroxysmal positional vertigo: A canalith jam? Neurological Sciences, 42, 313-315.
- Castellucci, A., Malara, P., Martellucci, S., Delmonte, S., & Ghidini, A. (2021). Fluctuating posterior canal function in benign paroxysmal positional vertigo depending on how and where otoconia are disposed. Otology & Neurotology, 42, e193-e198.
- Castellucci, A., Piras, G., Del Vecchio, V., Ferri, G. G., Ghidini, A., & Brandolini, C. (2021). Which inner ear disorders lie behind a selective posterior semicircular canal hypofunction on video head impulse test? Otology & Neurotology, 42, 573-584.
- Castellucci, A., Malara, P., Martellucci, S., Armato, E., & Califano, L. (2021). Possible pathomechanism behind the transient hypofunction of the affected canal in BPPV. European Archives of Oto-Rhino-Laryngology, 279(2), 1117-1118.
- Chang, Y. S., Choi, J., & Chung, W. H. (2014). Persistent direction-fixed nystagmus following canalith repositioning maneuver for horizontal canal BPPV: A case of canalith jam. Clinical and Experimental Otorhinolaryngology, 7(2), 138-141.
- Choi, J. Y., Kim, H. J., & Kim, J. S. (2018). Recent advances in head impulse test findings in central vestibular disorders. Neurology, 90, 602-612.
- S.-Y. Choi, M.-J. Lee, E.H. Oh, J.-H. Choi, and K.-D. Choi, Short-Term Central Adaptation in Benign Paroxysmal Positional Vertigo, Front Neurol 11 (2020), 260.
- Çınar, Y., Bayram, A., Culfa, R., & Mutlu, C. (2018). Analyses with the video head impulse test during the canalith repositioning maneuver in patients with isolated posterior semicircular canal benign paroxysmal positional vertigo. Turkish Archives of Otorhinolaryngology, 56, 81-84.
- Cocchi, C., Castellucci, A., Malara, P., Martellucci, S. & Ghidini, A. Spontaneous Reversing Positional Nystagmus as a sign of simultaneous ampullary and non-ampullary Benign Paroxysmal Positional Vertigo of the Posterior Semicircular Canal. J Vestib Res (under review)
- Elsherif, M., Eldeeb, D., & Eldeeb, M. (2021). Clinical significance of video head impulse test in benign paroxysmal positional vertigo: A meta-analysis. European Archives of Oto-Rhino-Laryngology, 278(12), 4645-4651.
- Epley, J. M. (1997). Caveats in particle repositioning for treatment of canalithiasis (BPPV). Operative Techniques in Otolaryngology-Head and Neck Surgery, 8, 868-876.
- Epley, J. M. (2001). Human experience with canalith repositioning maneuvers. Annals of the New York Academy of Sciences, 942, 179-191.
- Fallahnezhad, T., Adel Ghahraman, M., Farahani, S., Hoseinabadi, R., & Jalaie, S. (2017). Vestibulo-ocular reflex abnormalities in posterior semicircular canal benign paroxysmal positional vertigo: A pilot study. Iranian Journal of Otorhinolaryngology, 29, 269-274.
- S.-H. Lee, M.-K. Kim, K.-H. Cho, and J.S. Kim, Reversal of initial positioning nystagmus in benign paroxysmal positional vertigo involving the horizontal canal, Ann N Y Acad Sci 1164 (2009), 406–408.
- X. Li, L. Si, N. Song, Y. Wu, M. Zhang, Y. Feng, and X. Yang, Characteristics and Possible Mechanisms of Direction-Reversing Nystagmus During Positional Testing in Patients With Benign Paroxysmal Positional Vertigo, Otol Neurotol 44 (2023), e512–e518.
- Halmagyi, G. M., Chen, L., MacDougall, H. G., Weber, K. P., McGarvie, L. A., & Curthoys, I. S. (2017). The video head impulse test. Frontiers in Neurology, 8, 258.
- Luis, L., Costa, J., Vaz Garcia, F., Valls-Solé, J., Brandt, T., & Schneider, E. (2013). Spontaneous plugging of the horizontal semicircular canal with reversible canal dysfunction and recovery of vestibular evoked myogenic potentials. Otology & Neurotology, 34, 743-747.
- Mangabeira Albernaz, P. L., & Zuma e Maia, F. C. (2014). The video head impulse test. Acta Oto-Laryngologica, 134, 1245-1250.
- Martellucci, S., Castellucci, A., Malara, P., Pagliuca, G., Clemenzi, V., Stolfa, A., Gallo, A., & Libonati, G. A. (2022). Spontaneous jamming of horizontal semicircular canal combined with canalolithiasis of contralateral posterior semicircular canal. Journal of Audiology and Otology, 26(1), 55-60.
- D. Nuti, P. Vannucchi, and P. Pagnini, Benign paroxysmal positional vertigo of the horizontal canal: a form of canalolithiasis with variable clinical features, J Vestib Res 6 (1996), 173–184
- Y. Ogawa, A. Ichimura, K. Otsuka, A. Hagiwara, T. Inagaki, S. Shimizu, N. Nagai, S. Itani, and M. Suzuki, Spontaneous inversion of nystagmus without a positional change in the horizontal canal variant of benign paroxysmal positional vertigo, J Vestib Res 25 (2015), 169–175.
- P. Pagnini, D. Nuti, and P. Vannucchi, Benign paroxysmal vertigo of the horizontal canal, ORL J Otorhinolaryngol Relat Spec 51 (1989), 161–170.
- Saltürk, Z., & Yetişer, S. (2020). Video head impulse testing in patients with benign paroxysmal positional vertigo. Acta Oto-Laryngologica, 140(12), 977-981.
- Schubert, M. C., Helminski, J., Zee, D. S., Cristiano, E., Giannone, A., Tortoriello, G., & Marcelli, V. (2020). Horizontal semicircular canal jam: Two new cases and possible mechanisms. Laryngoscope Investigative Otolaryngology, 5, 163-167.
- J. Stahle and J. Terins, Paroxysmal positional nystagmus; an electronystagmographic and clinical study, Ann Otol Rhinol Laryngol 74 (1965), 69–83.
- Vannucchi, P., Pecci, R., & Giannoni, B. (2012). Posterior semicircular canal benign paroxysmal positional vertigo presenting with torsional downbeating nystagmus: An apogeotropic variant. International Journal of Otolaryngology, 2012, 413603.
- Vannucchi, P., Pecci, R., Giannoni, B., Di Giustino, F., Santimone, R., & Mengucci, A. (2015). Apogeotropic posterior semicircular canal benign paroxysmal positional vertigo: Some clinical and therapeutic considerations. Audiology Research, 5, 130.
- Vats AK, Vats S, Kothari S. Bilaterally Positive Dix-Hallpike Test (DHT) with Unilateral Direction-Reversing Positional Nystagmus in Patient with Apogeotropic Posterior Canal BPPV. Ann Indian Acad Neurol. 2023;26(6):1015-1016
- Vats AK, Vats S, Kothari S, Aswani N. Atypical Benign Paroxysmal Positional Vertigo: Concomitant Posterior Semicircular Cupulolithiasis with Ipsicanal Nonampullary Arm Posterior Semicircular Canalolithiasis. Ann Indian Acad Neurol. 2024;27(5):610-612.
- Von Brevern, M., Clarke, A. H., & Lempert, T. (2001). Continuous vertigo and spontaneous nystagmus due to canalolithiasis of the horizontal canal. Neurology, 56(5), 684-686.
- Von Brevern M, Bertholon P, Brandt T, Fife T, Imai T, Nuti D, Newman-Toker D (2015). Benign paroxysmal positional vertigo: Diagnostic criteria. J Vestib Res, 25(3-4):105-17.
- Yetiser S. A New Variant of Posterior Canal Benign Paroxysmal Positional Vertigo: A Nonampullary or Common Crus Canalolithiasis. Case Rep Otolaryngol. 2015;2015:816081
- Yetiser S. Spontaneous Direction-Changing or Reversing Positional Nystagmus without Changing Head Position during Head-Roll/Head-Hanging Maneuvers: Biphasic Positional Nystagmus. J Audiol Otol. 2021;25(1):43-48.
Resources for More Information
For more information, visit https://www.inventis.it/en-na


