Inner Ear Disorders that Lie Behind a Selective Canal Impairment on the Video-HIT
Introduction
It is well known that vestibular sensors include three semicircular canals (SCs), i.e., horizontal (HSC), anterior (ASC), posterior (PSC), and two otolithic organs: the saccule and the utricle. The recent introduction of modern devices assessing ampullary and otolith reflexes in the high-frequency domain (video-HIT for the three ampullary afferents, cervical VEMPs, and ocular VEMPs for saccular and utricular afferents, respectively) has enabled a fast, functional analysis of all inner ear receptors. This allows for new interpretations of abnormalities involving end-organs and afferents that can be detected in patients with cochleovestibular pathologies in both acute and chronic stages. In fact, case series and investigations on study cohorts have demonstrated how vestibular disorders can result in specific lesion patterns depending on the lesion site, the underlying pathomechanism, and the related etiology.
To better understand the reasons behind a selective SC involvement, a brief recall of anatomical principles is needed. The labyrinthine afferents run through the vestibular nerve, that is composed of two branches: the superior and the inferior vestibular nerve (IVN). While the former is mainly composed of the lateral and anterior ampullary nerves and the utricular nerve (with a possible afferent coming from the anterosuperior portion of the saccule), the IVN is composed of the posterior ampullary nerve (or singular nerve) and the saccular nerve. Basically, the same dichotomy of “superior” and “inferior” labyrinth is also preserved in the arterial vascularization of the labyrinth. In fact, the inner ear is supplied by the internal auditory artery, which divides into two main terminal branches: the anterior vestibular artery and the common cochlear artery (CCA). Whereas the first mostly supplies the utricle and both HSC and ASC, the latter mainly serves the saccule, PSC, and the cochlea, with slight possible interindividual differences. In turn, the CCA divides into the vestibulocochlear artery, which serves PSC, the saccule, and the cochlear basal turn, and in the main cochlear artery supplying the rest of the cochlear neuroepithelium. Finally, the posterior vestibular artery generates from the vestibulo-cochlear artery, providing blood supply to both PSC and saccule.
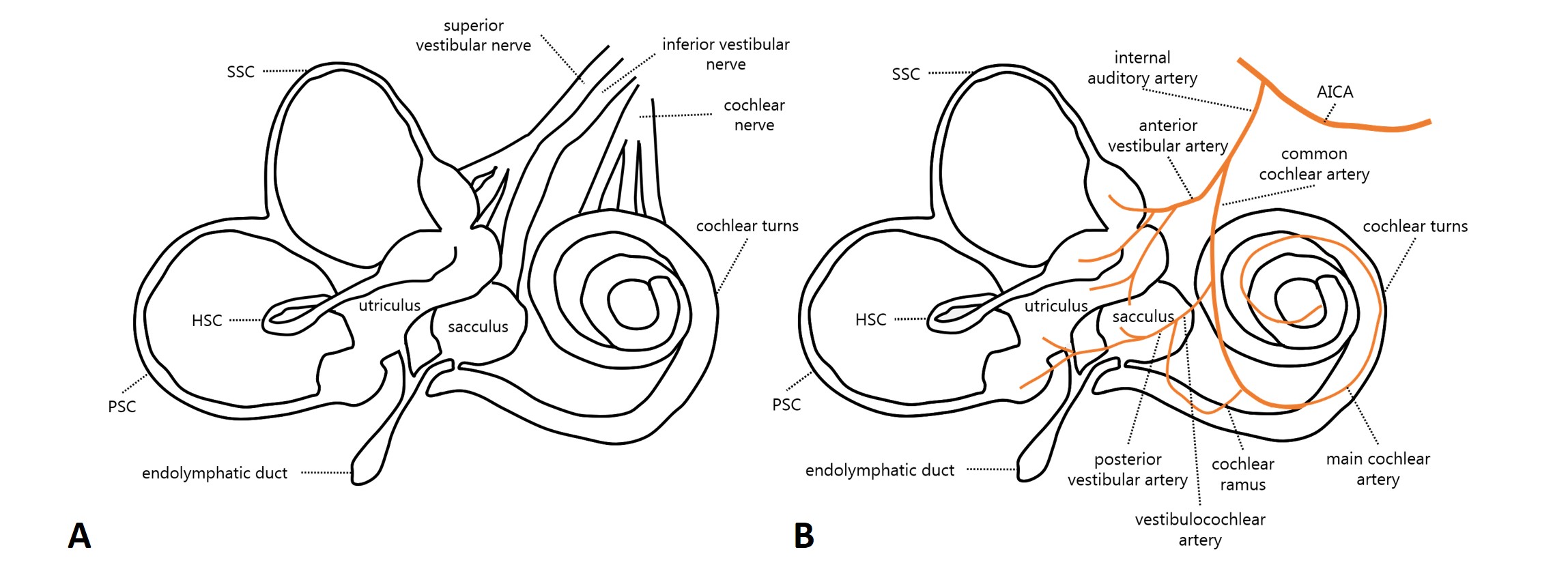
Figure 1. (A) Labyrinthine innervation (modified from Schuknecht HF, 1993) and (B) inner ear arterial supply (modified from Kim JS & Lee H, Semin Neurol 2009).
AudiologyOnline: Which is the most frequently involved semicircular canal on video-HIT, and which are the underlying pathomechanisms accounting for a selective canal impairment?
Andrea Castellucci: Given the aforementioned conditions, it is easy to understand how the PSC represents the most frequently involved SC in isolation on the video-HIT, as both acute and chronic diseases affecting the neural pathways, such as vestibular neuritis and vestibular schwannoma, respectively, can give an isolated SC impairment mostly when involving the IVN (resulting in PSC and saccular dysfunction) and, similarly, acute ischemic lesions of the labyrinth can result in a selective SC dysfunction mostly when the CCA or its branches represent the lesion sites (resulting in PSC and saccular involvement in association with sudden hearing loss). While in both cases, the saccular function should be damaged, resulting in cervical VEMPs impairment, acute vestibular symptoms should be associated with sudden sensorineural hearing loss (SSNHL) only in ischemic lesions, whereas the cochlear function is spared in inferior vestibular neuritis.
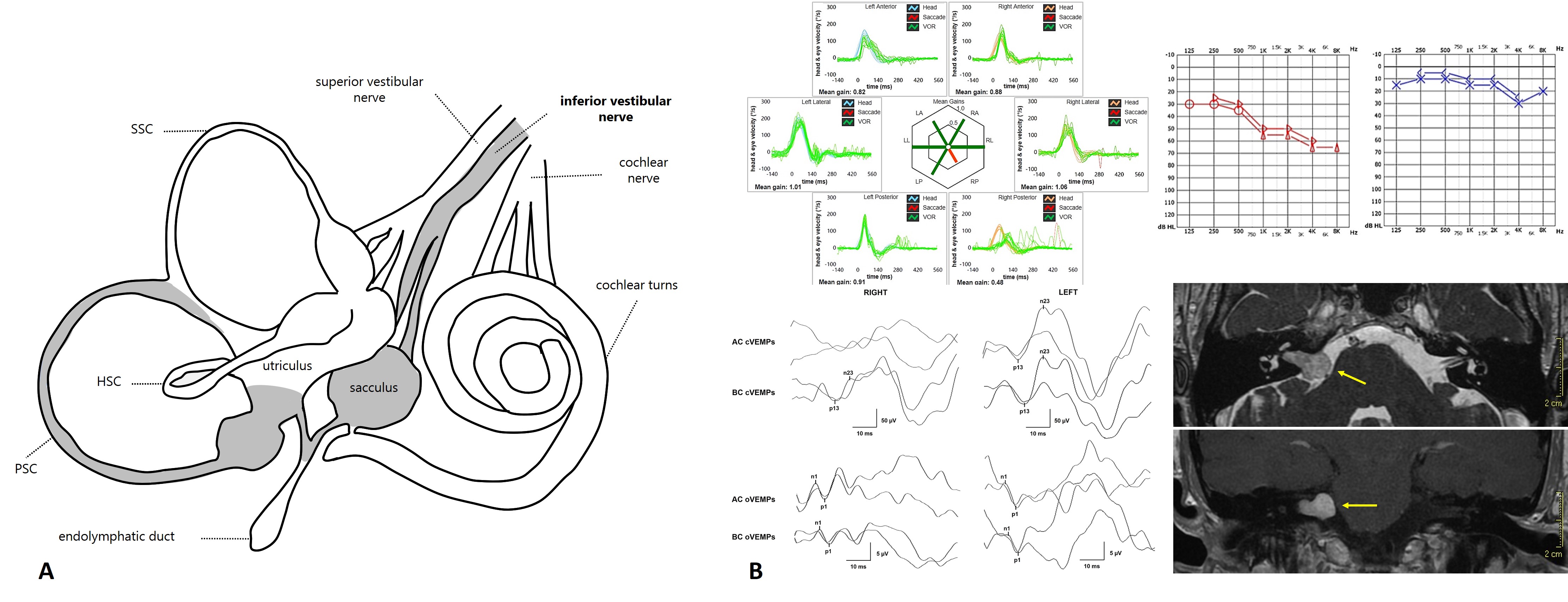
Figure 2. (A) Inner ear structures involved in inferior vestibular neuritis (from Comacchio C & Castellucci A, Front Neurol, 2022). (B) Vestibular schwannoma growing from the IVN and related instrumental pattern (from Castellucci A et al., Otol Neurotol. 2021).
Depending on the ischemic arterial branch, cochlear lesion could result either in profound SSNHL due to an ischemia of all cochlear turns (in case of CCA infarction) or in high-frequency SSNHL likely due to an infarction in the territory supplied by the vestibulocochlear artery (basal turns). Clinicians should also be aware that the instrumental picture due to an infarction in the territory supplied by the posterior vestibular artery perfectly overlaps an inferior vestibular neuritis, as the cochlear blood supply is spared in this case. This condition likely represents the only exception to the rule that inner ear vascular lesions with PSC impairment are always associated with SSNHL.
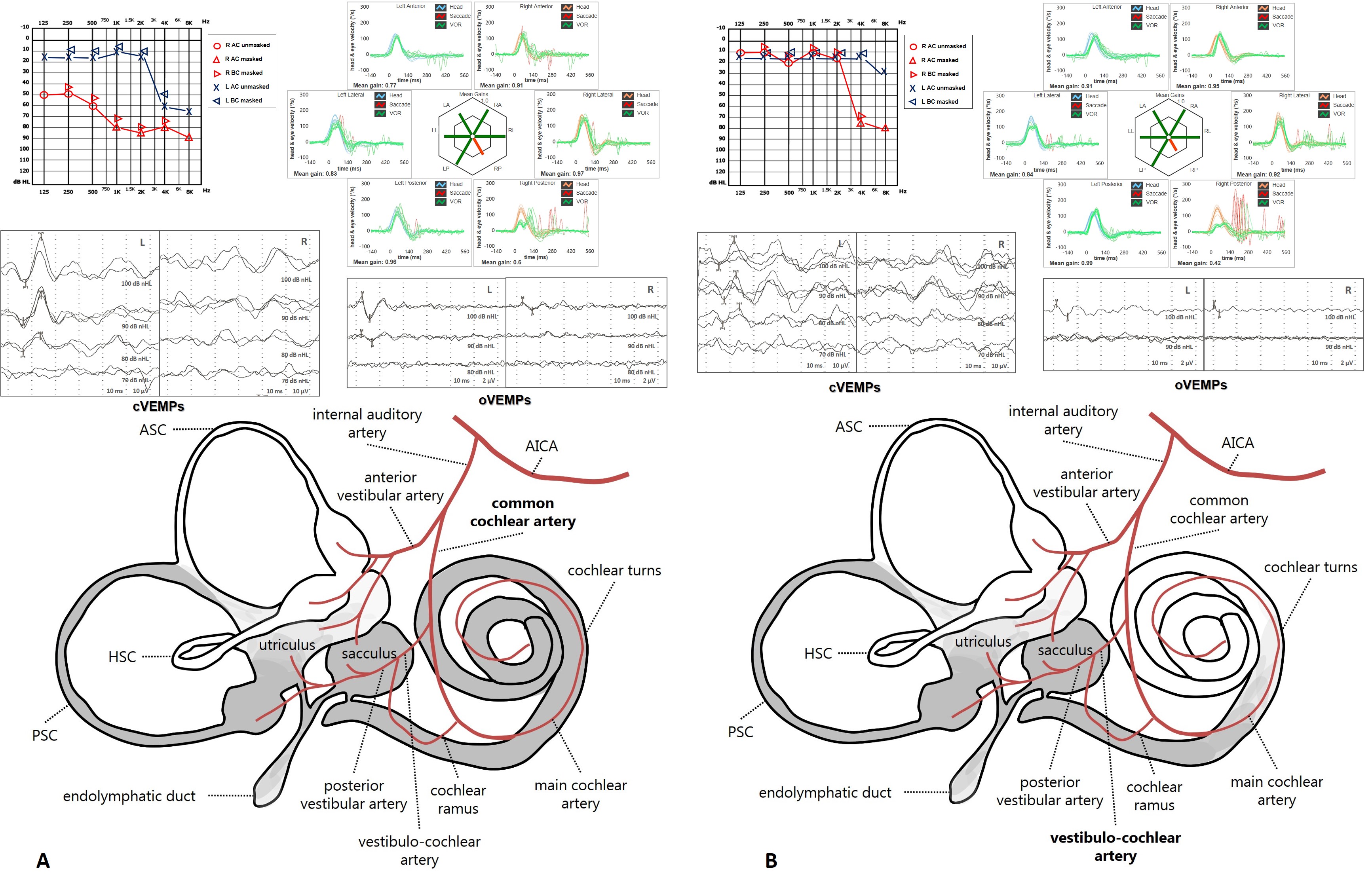
Figure 3. Labyrinthine ischemia and related instrumental patterns due to (A) CCA occlusion and (B) vestibulocochlear artery involvement (from Castellucci A et al., Front Neurol. 2023).
Moreover, we should also consider that the PSC lies in the undermost part of the labyrinth when the patient is in both an upright and supine position, enhancing its vulnerability to chronic damages on a hydropic basis and acute dysfunctions due to dislodged otoconia. Both conditions can result in canal VOR-gain hypofunction at the video-HIT through different mechanisms.
Less commonly, the video-HIT can detect an isolated abnormal VOR gain for the other SCs. For example, in case of an acute unilateral vestibular loss (AUVL), a selective impairment either for the HSC or for the ASC could be found mostly in the recovery stage following a superior vestibular neuritis or an anterior vestibular artery ischemia (where the damaged receptors are both HSC and ASC, together with the utricle in both cases), likely due to an asynchronous/asymmetrical recovery process between the two SCs and/or to different central compensatory mechanisms.
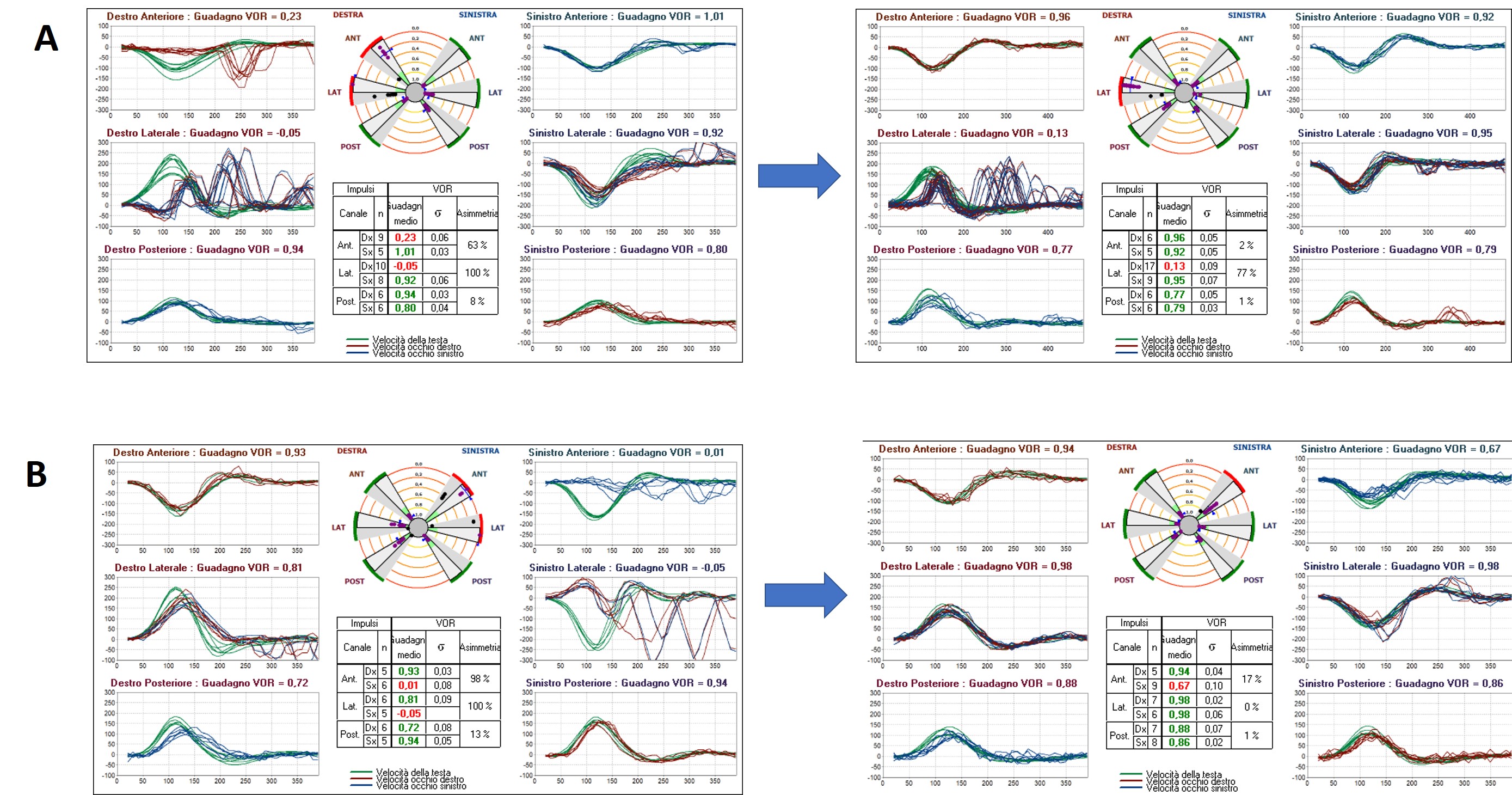
Figure 4. video-HIT measurements in acute and post-acute stage of an AUVL on the right (A) and left side (B) where an asynchronous recovery between the HSC and ASC occur. Presenting scenario could be due either due to a superior vestibular neuritis or an anterior vestibular artery ischemia.
On the other hand, both Menière’s disease (MD) in its different stages and benign paroxysmal positional vertigo (BPPV) can result in different patterns of selective SCs involvement depending on the site of the inner ear that is mostly affected by endolymphatic hydrops (EH) and on how and where otoliths are disposed, respectively.
In fact, MD represents an idiopathic fluctuating inner ear disorder likely due to EH leading to episodic vertigo spells variably associated with tinnitus and hearing loss. Likewise, low-frequency hearing fluctuations, and rapid fluctuations of vestibular function have also been documented with video-HIT and VEMPs in MD during the attack, and different lesion patterns have been reported according to the different stages of the disease (early-stage vs. late-stage) and to the possible inner ear compartments involved by EH. Several pathomechanisms might occur in patients with MD; they include the ionic - chemical perilymphatic intoxication, the endolymphatic space collapse due to membranes rupture, both acute and chronic fluid pressure changes, and saccular/utricular herniation into the SCs. All these phenomena can variably result in either transient or persistent hair cells dysfunction and in alterations of the dynamic property of the peripheral vestibular system leading either to fluctuation of SC and otolith activity during MD attacks or progressive impairment of vestibular function. Depending on the area affected by EH and the aforementioned phenomena, it might be possible to detect a selective lesion of an SC on video-HIT, variably associated with different results at the rest of the instrumental test battery. For example, it is possible to observe in the acute stage either irritative or paretic spontaneous horizontal nystagmus associated with impaired HSC VOR-gain at the video-HIT, normal or reduced cervical VEMPs, and enhanced ocular VEMPs for the affected side. In some other cases, it is possible to observe an ictal spontaneous downbeat nystagmus with slight contralesional torsional components associated with reduced PSC function and cervical VEMPs for the affected ear. Anecdotally, it has been reported the onset of spontaneous upbeat nystagmus with reduced ASC VOR-gain on video-HIT for the affected side during the acute stage of MD.
Usually, all these findings tend to normalize as soon as the ictal stage ceases. In chronic stages of MD, PSC tends to impair over time, as well as both cochlear and otolith function.
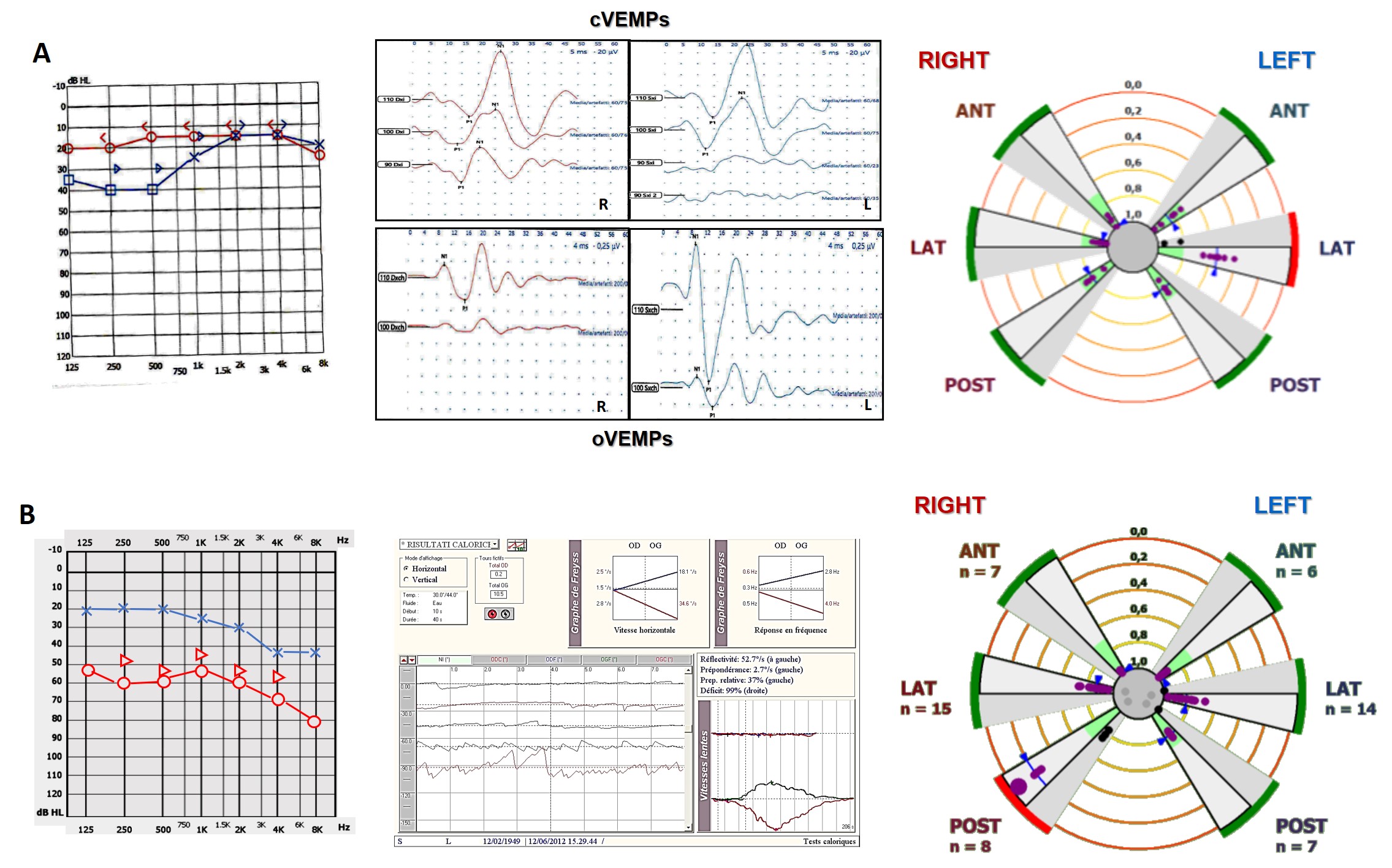
Figure 5. (A) Ictal stage in a patient with left-sided MD with related instrumental pattern. (B) Inter-ictal/chronic stage in a patient with right-sided MD with related instrumental pattern.
Conversely, BPPV can result in SC impairment only in selected conditions, as free-floating otoconia leading to paroxysmal nystagmus with crescendo-decrescendo course usually do not alter high-frequency responses of the affected SC, preserving video-HIT measurements. On the contrary, in case otoliths are completely or incompletely entrapped in narrow tracts of the membranous ducts, dynamic responses of the affected SC might be impaired, resulting in challenging differential diagnosis with AUVL or with central nervous system (CNS) disorders. According to the recent literature, these situations include the canalith jam and those ASC-BPPV and apogeotropic variants of PSC-BPPV with persistent positional downbeat nystagmus (DBN), where an incomplete (or positional) canalith jam could be hypothesized. In the first case, an otolith clot is thought to entirely plug the canal (either spontaneously or as a result of inappropriate repositioning procedures) and prevent endolymphatic flows resulting in a continuous positive or negative pressure between the clot itself and the cupula. This condition may likely result in a persistent deflection of the cupula, accounting for spontaneous nystagmus irrespective to head positions. Depending on the SC affected, spontaneous nystagmus might be either horizontal, mimicking an AUVL, or vertical/torsional, mimicking CNS disorders. In these situations, both high- and low-frequency responses for the affected SC should be impaired.
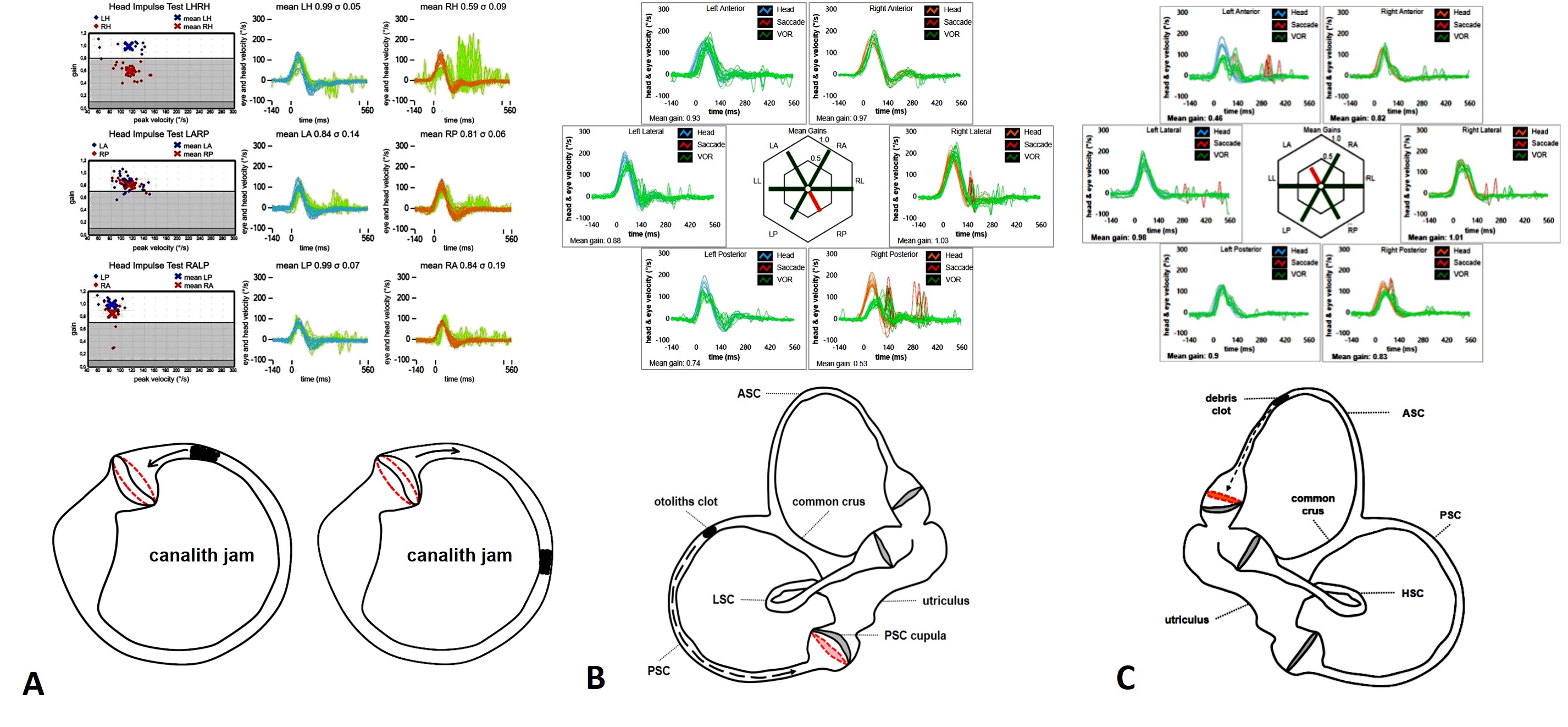
Figure 6. Complete canalith jam involving either the right HSC (A) (from Castellucci et al. Am J Otolaryngol. 2019), the right PSC (B) (from Castellucci et al. Neurol Sci. 2021), and the left ASC (C) (from Castellucci et al. J Audiol Otol, 2022) with related video-HIT measurements.
In the second condition, otoliths are supposed to be only partially entrapped in a stenotic tract of the ASC (ASC-BPPV) or settle in the distal portion of the non-ampullary arm of the PSC, close to the common crus (“apogeotropic variant” of PSC-BPPV), generating a persistent positional DBN in provoking positionings (straight head hanging and/or Dix Hallpike positions). In fact, whereas in ASC-BPPV, debris are thought to shift away from ASC ampulla resulting in a utriculofugal deflection of the cupula and in an excitatory discharge of the superior ampullary nerve, in apogeotropic PSC-BPPV positioning maneuvers should move debris towards the ampulla, producing an inhibition of PSC afferents. In particular, in cases with persistent positional DBN, where a partial otolith entrapment is more likely to occur than in cases with paroxysmal transient positional DBN, an “incomplete” or “positional” jam behaving as a “low-pass filter” has been hypothesized, i.e., allowing the cupula to be activated by low-frequency stimuli (otoconial shifts) while impeding the ampullary receptor to respond to high-frequency inputs (head impulses). This pathomechanism has allowed explaining how patients with persistent positional DBN due to ASC-BPPV or apogeotropic PSC-BPPV exhibited impaired VOR-gain values for the affected SC, restoring after repositioning maneuvers or after conversion in a classical ipsilateral BPPV with paroxysmal nystagmus.
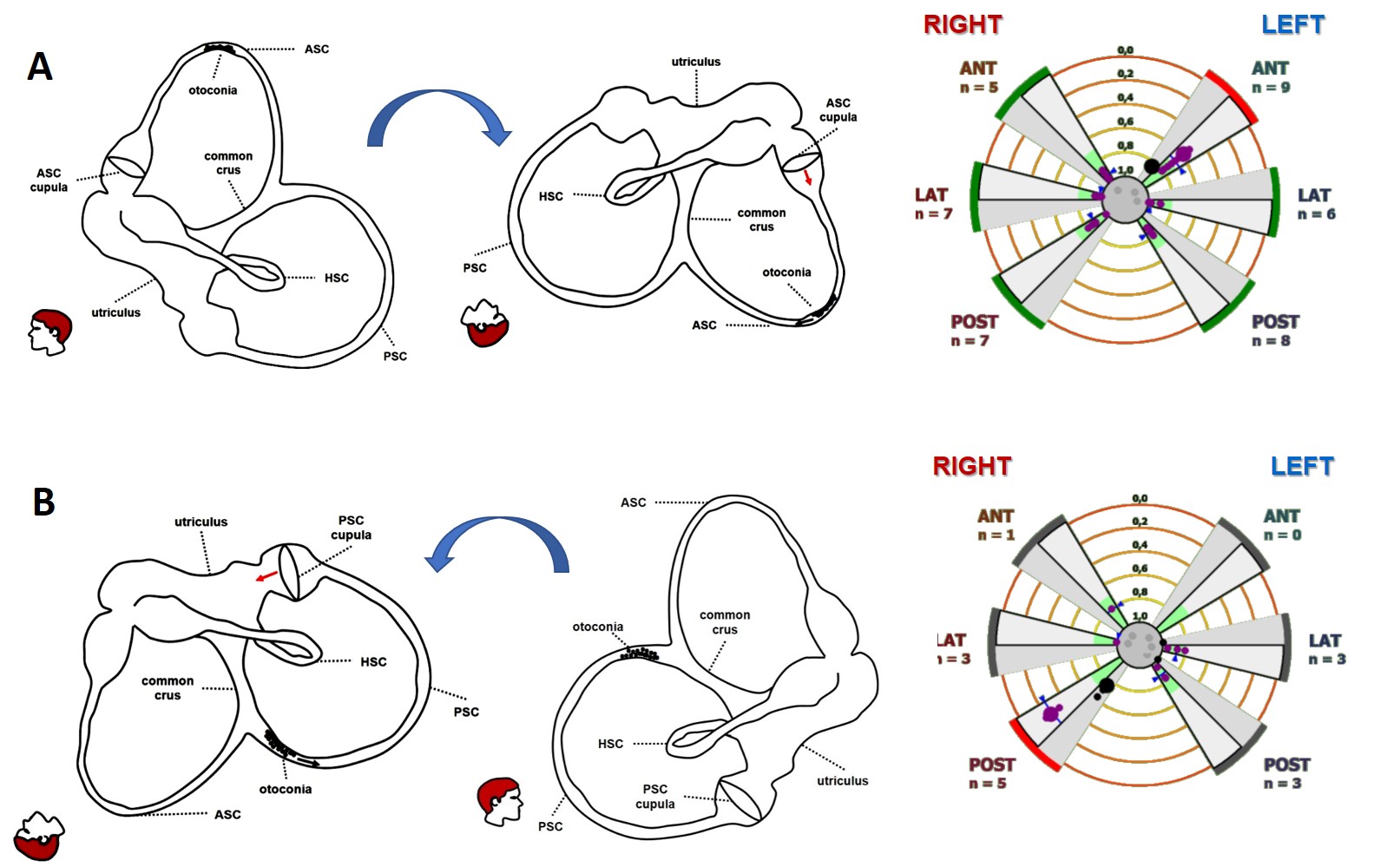
Figure 7. Incomplete canalith jam resulting in left ASC-BPPV (A) and right apogeotropic PSC-BPPV (B) (from Castellucci et al. Front Neurol. 2020).
Finally, since wide-sized bony dehiscences have demonstrated to result in a functional impairment in the high-frequency domain for the involved SC, it is possible to detect a selective hypofunction of a dehiscent SC on video-HIT depending on the affected SC. According to the literature, the ASC (also known as superior SC - SSC) is the far more frequently affected structure, while the PSC has been less often reported and the HSC only anecdotally. In case of particularly wide bony defect, the content of the surrounding structures (middle fossa in case of SSC dehiscence, while posterior fossa or high-riding jugular bulb for PSC dehiscence) might herniate into the canal through the dehiscence and spontaneously plug the membranous duct, excluding the SC from labyrinthine function by impeding the flow of endolymph. Conversely, it has been recently hypothesized how particularly large dehiscence might allow a dissipation of mechanical energy through the dehiscence during high-acceleration movements. This condition could result in a reduced amount of fluid-mechanical waves efficiently pulling the SC cupula away from the utricle to excite canal afferents during head impulse, thus accounting for impaired VOR-gain values on video-HIT.
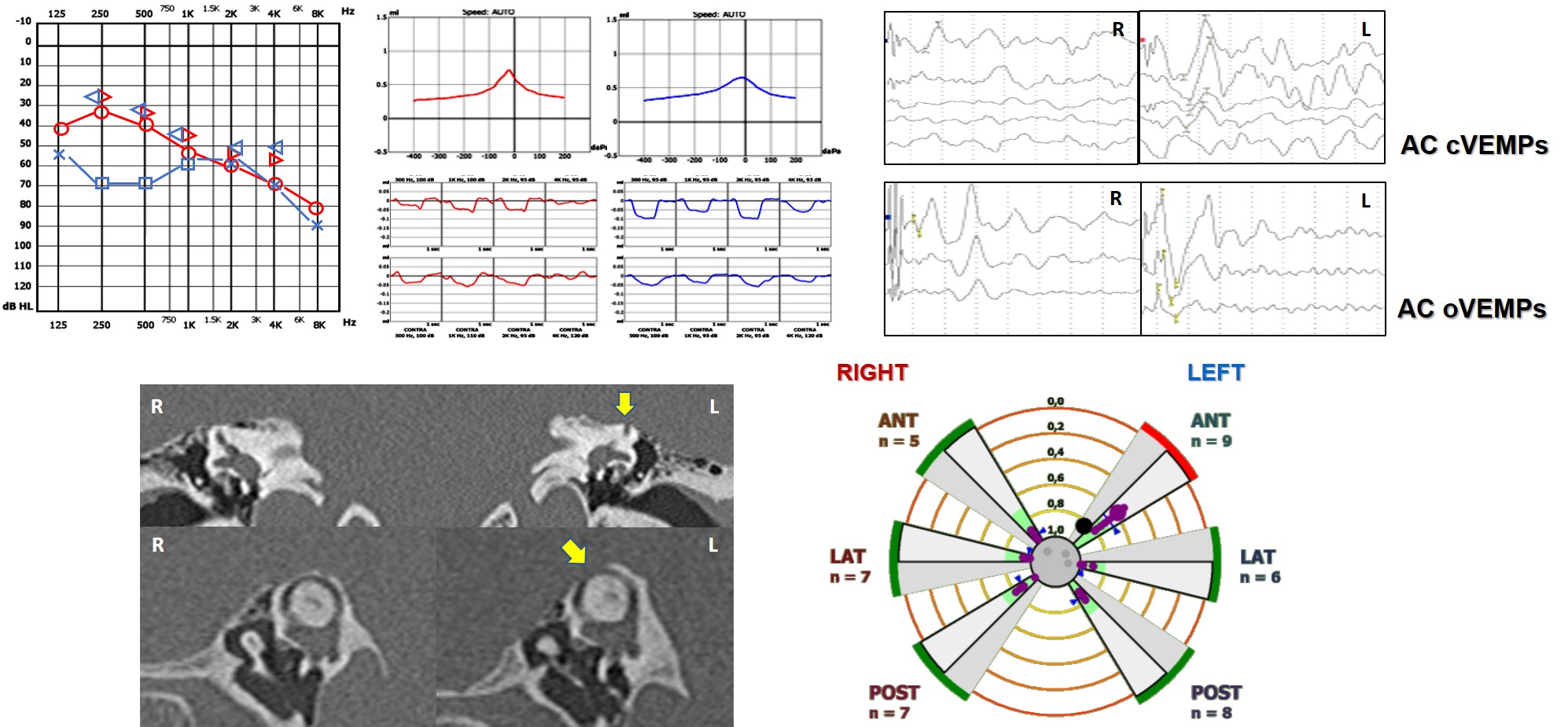
Figure 8. Left wide-sized SSCD with related instrumental assessment.
AudiologyOnline: Which are the peripheral disorders of the inner ear underlying an HSC impairment on video-HIT?
Andrea Castellucci: Below, a schematic overview of the possible diseases leading to selective impairment for the HSC on video-HIT in relation with the remaining clinical-instrumental data, including VOG findings, cervical/ocular VEMPs, caloric test, and audiometry.
Selective HSC loss on video-HIT | ||||||
disease | VOG | audiometry | cervical VEMPs | ocular VEMPs | caloric test | assumed pathomechanisms |
MD (ictal stage) | spontaneous ipsilesional (“irritative”) or contralesional (“paretic”) horizontal nystagmus | normal or low-frequency hearing loss | normal or reduced +/- frequency tuning | enhanced | canal paresis / normal values | hydrops in the membranous labyrinth and/or fluid pressure changes and/or ionic - chemical perilymphatic intoxication might lead to increased mechanical resistance and/or transient hair cells dysfunction |
MD (inter-ictal / chronic stage) | spontaneous contralesional (“paretic”) or ipsilesional (“recovery”) horizontal nystagmus or nystagmus elicited by head shakings and/or skull vibrations | normal or low-frequency hearing loss or flat hearing loss | reduced +/- frequency tuning | normal or reduced | canal paresis | hydrops in the membranous labyrinth, increased mechanical resistance, endolymphatic space collapse due to membrane rupture, and utricular herniation into the HSC might lead to chronic hair cells dysfunction |
HSC BPPV (complete canalith jam) | spontaneous contralesional (“paretic”) or ipsilesional (“irritative”) horizontal nystagmus | normal | normal | normal | canal paresis | an otolith clot entrapped within the membranous duct of the HSC is thought to exert spontaneously a continuous pressure (either negative or positive) on the cupula, persistently inhibiting or exciting HSC afferents, respectively |
previous AUVL (superior vestibular neuritis or labyrinthine ischemia) | contralesional “paretic” horizontal nystagmus induced by head shakings and/or skull vibrations | normal | normal | reduced or normal | canal paresis | asymmetrical and/or asynchronous recovery of peripheral afferents/sensors and/or different central compensatory mechanisms |
AudiologyOnline: Which are the peripheral disorders of the inner ear underlying an ASC impairment on video-HIT?
Andrea Castellucci: Below, a schematic overview of the possible diseases leading to selective impairment for the ASC on video-HIT in relation with the remaining clinical-instrumental data, including VOG findings, cervical/ocular VEMPs, and audiometry.
Selective ASC loss on video-HIT | |||||
disease | VOG | audiometry | cervical VEMPs | ocular VEMPs | assumed pathomechanisms |
SSC dehiscence | vertical/torsional nystagmus induced by Valsalva maneuvers and/or by, loud sounds and/or skull vibrations | normal or low-frequency conductive hearing loss with preserved acoustic reflexes | enhanced amplitudes with lowered thresholds | enhanced amplitudes with lowered thresholds | auto-plugging process due to herniation of middle fossa content into the canal or dissipation of mechanical energy through the dehiscence during head impulses |
MD (ictal stage) | spontaneous upbeat nystagmus with contralesional (“paretic”) torsional components or nystagmus evoked by head shakings or skull vibrations | normal or low-frequency hearing loss | reduced +/- frequency tuning | normal or enhanced | hydrops in the membranous labyrinth and/or fluid pressure changes and/or ionic - chemical perilymphatic intoxication might lead to increased mechanical resistance and/or transient hair cells dysfunction |
ASC-BPPV (incomplete canalith jam) | persistent positional DBN in Dix Hallpike and/or straight head hanging positions with variable torsional components | normal | normal | normal | debris are partially entrapped in the ASC and are thought to exert a continuous negative pressure on the cupula in provoking positionings, exciting ASC afferents |
ASC-BPPV (complete canalith jam) | upbeat spontaneous nystagmus with variable torsional components | normal | normal | normal | debris is completely entrapped in the membranous duct and are thought to exert spontaneously a continuous positive pressure on the cupula, persistently inhibiting ASC afferents |
previous AUVL (superior vestibular neuritis or labyrinthine ischemia) | upbeat nystagmus with contralesional (“paretic”) torsional components evoked by head shakings and/or skull vibrations | normal | normal | reduced or normal | asymmetrical and/or asynchronous recovery of peripheral afferents and/or different central compensatory mechanisms |
AudiologyOnline: Which are the peripheral disorders of the inner ear underlying a PSC impairment on video-HIT?
Andrea Castellucci: Below, a schematic overview of the possible diseases leading to selective impairment for the PSC on video-HIT in relation with the remaining clinical-instrumental data, including VOG findings, cervical/ocular VEMPs, and audiometry.
Selective PSC loss on video-HIT | |||||
disease | VOG | audiometry | cervical VEMPs | ocular VEMPs | assumed pathomechanisms |
Labyrinthine ischemia in the CCA territory | variable spontaneous DBN with contralesional torsional components | flat / down-sloping SSNHL | reduced / absent | normal / reduced | acute damage of the inner ear sensors supplied by the CCA and its branches (i.e. cochlea, saccule, PSC) |
Inferior vestibular neuritis | spontaneous DBN with contralesional torsional components | normal | reduced / absent | normal | acute loss of PSC and saccular afferent running through the IVN |
Vestibular schwannoma | nystagmus induced by skull vibrations and/or head shakings and/or hyperventilation test | sensorineural hearing loss or normal hearing function | reduced / absent | normal | progressive impairment of PSC and saccular afferents running through the IVN associated with tumor compression on cochlear artery |
MD (ictal stage) | spontaneous DBN with contralesional (“paretic”) torsional components or nystagmus evoked by skull vibrations and/or head shakings | normal or low-frequency hearing loss | reduced +/- frequency tuning | enhanced or reduced | hydrops in the membranous labyrinth and/or fluid pressure changes and/or ionic - chemical perilymphatic intoxication might lead to increased mechanical resistance and/or transient hair cells dysfunction |
MD (inter-ictal stage) | DBN with contralesional (“paretic”) torsional components evoked by skull vibrations and/or head shakings | normal or low-frequency or flat hearing loss | reduced +/- frequency tuning | normal or reduced | hydrops in the membranous labyrinth, increased mechanical resistance, endolymphatic space collapse due to membrane rupture, and saccular herniation into the PSC might lead to chronic hair cells dysfunction |
PSC-BPPV (incomplete canalith jam) | persistent positional DBN in Dix Hallpike and/or straight head hanging positions with variable torsional components | normal | normal | normal | debris are partially entrapped in the non-ampullary arm of the PSC and are thought to exert a continuous positive pressure on the cupula in provoking positionings, inhibiting PSC afferents |
PSC-BPPV (complete canalith jam) | spontaneous DBN with contralesional (“paretic”) torsional components | normal | normal | normal | debris is completely entrapped in the membranous duct and are thought to exert spontaneously a continuous positive pressure on the cupula, persistently inhibiting PSC afferents |
PSC dehiscence | vertical/torsional nystagmus induced by Valsalva maneuvers and/or by, loud sounds, and/or by skull vibrations | normal or low-frequency conductive HL with preserved acoustic reflexes | enhanced amplitudes with lowered thresholds | enhanced amplitudes with lowered thresholds | auto-plugging process due to herniation of posterior fossa content or high-riding jugular bulb into the canal or dissipation of mechanical energy through the dehiscence during head impulses |
Resources for More Information
For more information, visit https://www.inventis.it/en-na or view this AudiologyOnline course, Selective Canal Impairment on Video-HIT in Peripheral Vestibular Diseases.
References
Allum, J. H. J., & Honegger, F. (2020). Improvement of asymmetric vestibulo-ocular reflex responses following onset of vestibular neuritis is similar across canal planes. Frontiers in Neurology, 11, 565125. https://doi.org/10.3389/fneur.2020.565125
Aw, S. T., Fetter, M., Cremer, P. D., Karlberg, M., & Halmagyi, G. M. (2001). Individual semicircular canal function in superior and inferior vestibular neuritis. Neurology, 57(5), 768–774. https://doi.org/10.1212/wnl.57.5.768
Bartolomeo, M., Biboulet, R., Pierre, G., Mondain, M., Uziel, A., & Venail, F. (2014). Value of the video head impulse test in assessing vestibular deficits following vestibular neuritis. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery, 271(4), 681–688. https://doi.org/10.1007/s00405-013-2451-y
Büki, B., Hanschek, M., & Jünger, H. (2017). Vestibular neuritis: Involvement and long-term recovery of individual semicircular canals. Auris, Nasus, Larynx, 44(3), 288–293. https://doi.org/10.1016/j.anl.2016.07.020
Califano, L., Iannella, R., Mazzone, S., Salafia, F., & Melillo, M. G. (2021). The Video Head Impulse Test in the acute stage of posterior canal benign paroxysmal positional vertigo. Acta otorhinolaryngologica Italica : organo ufficiale della Societa italiana di otorinolaringologia e chirurgia cervico-facciale, 41(1), 69–76. https://doi.org/10.14639/0392-100X-N1033
Castellucci, A., Malara, P., Brandolini, C., Del Vecchio, V., Giordano, D., Ghidini, A., Ferri, G. G., & Pirodda, A. (2019). Isolated horizontal canal hypofunction differentiating a canalith jam from an acute peripheral vestibular loss. American Journal of Otolaryngology, 40(2), 319–322. https://doi.org/10.1016/j.amjoto.2018.12.005
Castellucci, A., Malara, P., Delmonte, S., & Ghidini, A. (2020). A possible role of video-head impulse test in detecting canal involvement in benign paroxysmal positional vertigo presenting with positional downbeat nystagmus. Otology & Neurotology: Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 41(3), 386–391. https://doi.org/10.1097/MAO.0000000000002500
Castellucci, A., Malara, P., Martellucci, S., Botti, C., Delmonte, S., Quaglieri, S., Rebecchi, E., Armato, E., Ralli, M., Manfrin, M. L., Ghidini, A., & Asprella Libonati, G. (2020). Feasibility of using the video-head impulse test to detect the involved canal in benign paroxysmal positional vertigo presenting with positional downbeat nystagmus. Frontiers in Neurology, 11, 578588. https://doi.org/10.3389/fneur.2020.578588
Castellucci, A., Malara, P., & Ghidini, A. (2021). Spontaneous downbeat nystagmus in posterior semicircular canal benign paroxysmal positional vertigo: a canalith jam?. Neurological Sciences: Official Journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, 42(1), 313–315. https://doi.org/10.1007/s10072-020-04529-9
Castellucci, A., Malara, P., Martellucci, S., Delmonte, S., & Ghidini, A. (2021). Fluctuating posterior canal function in benign paroxysmal positional vertigo depending on how and where otoconia are disposed. Otology & Neurotology: Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 42(2), e193–e198. https://doi.org/10.1097/MAO.0000000000002913
Castellucci, A., Piras, G., Del Vecchio, V., Crocetta, F. M., Maiolo, V., Ferri, G. G., Ghidini, A., & Brandolini, C. (2021). The effect of superior canal dehiscence size and location on audiometric measurements, vestibular-evoked myogenic potentials and video-head impulse testing. European Archives of Oto-Rhino-Laryngology: Official Journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery, 278(4), 997–1015. https://doi.org/10.1007/s00405-020-06169-3
Castellucci, A., Piras, G., Del Vecchio, V., Ferri, G. G., Ghidini, A., & Brandolini, C. (2021). Which inner ear disorders lie behind a selective posterior semicircular canal hypofunction on video head impulse test?. Otology & Neurotology: Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 42(4), 573–584. https://doi.org/10.1097/MAO.0000000000002995
Castellucci, A., Pepponi, E., Bertellini, A., Senesi, C., Bettini, M., Botti, C., Martellucci, S., Malara, P., Delmonte, S., Crocetta, F. M., Fornaciari, M., Lusetti, F., Bianchin, G., & Ghidini, A. (2021). Case report: Filling defect in posterior semicircular canal on MRI with balanced steady-state gradient-echo sequences after labyrinthine ischemia in the common cochlear artery territory as an early sign of fibrosis. Frontiers in Neurology, 11, 608838. https://doi.org/10.3389/fneur.2020.608838
Castellucci, A., Martellucci, S., Malara, P., Botti, C., Del Vecchio, V., Brandolini, C., Ferri, G. G., Ghidini, A., & Armato, E. (2021). Possible pathomechanisms accounting for both sound/pressure-induced eye movements and video head impulse test data in superior canal dehiscence. Acta Oto-Laryngologica, 141(8), 749–753. https://doi.org/10.1080/00016489.2021.1944664
Castellucci, A., Botti, C., Martellucci, S., Malara, P., Delmonte, S., Lusetti, F., & Ghidini, A. (2022). Spontaneous upbeat nystagmus and selective anterior semicircular canal hypofunction on video head impulse test: a new variant of canalith jam?. Journal of Audiology & Otology, 26(3), 153–159. https://doi.org/10.7874/jao.2021.00297
Castellucci, A., Botti, C., Delmonte, S., Bettini, M., Lusetti, F., Brizzi, P., Ruberto, R., Gamberini, L., Martellucci, S., Malara, P., Armato, E., Renna, L., Ghidini, A., & Bianchin, G. (2023). Vestibular assessment in sudden sensorineural hearing loss: Role in the prediction of hearing outcome and in the early detection of vascular and hydropic pathomechanisms. Frontiers in Neurology, 14. https://doi.org/10.3389/fneur.2023.1127008
Chihara, Y., Iwasaki, S., Murofushi, T., Yagi, M., Inoue, A., Fujimoto, C., Egami, N., Ushio, M., Karino, S., Sugasawa, K., & Yamasoba, T. (2012). Clinical characteristics of inferior vestibular neuritis. Acta Oto-Laryngologica, 132(12), 1288–1294. https://doi.org/10.3109/00016489.2012.701326
Comacchio, F., & Castellucci, A. (2022). Posterior semicircular canal ossification following acute vestibular loss mimicking inferior vestibular neuritis: A case report. Frontiers in Neurology, 13, 1015555. https://doi.org/10.3389/fneur.2022.1015555
Cordero-Yanza, J. A., Arrieta Vázquez, E. V., Hernaiz Leonardo, J. C., Mancera Sánchez, J., Hernández Palestina, M. S., & Pérez-Fernández, N. (2017). Comparative study between the caloric vestibular and the video-head impulse tests in unilateral Menière's disease. Acta Oto-Laryngologica, 137(11), 1178–1182. https://doi.org/10.1080/00016489.2017.1354395
Cremer, P. D., Halmagyi, G. M., Aw, S. T., Curthoys, I. S., McGarvie, L. A., Todd, M. J., Black, R. A., & Hannigan, I. P. (1998). Semicircular canal plane head impulses detect absent function of individual semicircular canals. Brain: A Journal of Neurology, 121( Pt 4), 699–716. https://doi.org/10.1093/brain/121.4.699
Fiorino, F., Pizzini, F. B., Beltramello, A., & Barbieri, F. (2011). Progression of endolymphatic hydrops in Ménière's disease as evaluated by magnetic resonance imaging. Otology & Neurotology: Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 32(7), 1152–1157. https://doi.org/10.1097/MAO.0b013e31822a1ce2
Fukushima, M., Oya, R., Nozaki, K., Eguchi, H., Akahani, S., Inohara, H., & Takeda, N. (2019). Vertical head impulse and caloric are complementary but react opposite to Meniere's disease hydrops. The Laryngoscope, 129(7), 1660–1666. https://doi.org/10.1002/lary.27580
Halmagyi, G. M., Aw, S. T., Karlberg, M., Curthoys, I. S., & Todd, M. J. (2002). Inferior vestibular neuritis. Annals of the New York Academy of Sciences, 956, 306–313. https://doi.org/10.1111/j.1749-6632.2002.tb02829.x
Kahn, L., Hautefort, C., Guichard, J. P., Toupet, M., Jourdaine, C., Vitaux, H., Herman, P., Kania, R., Houdart, E., Attyé, A., & Eliezer, M. (2020). Relationship between video head impulse test, ocular and cervical vestibular evoked myogenic potentials, and compartmental magnetic resonance imaging classification in menière's disease. The Laryngoscope, 130(7), E444–E452. https://doi.org/10.1002/lary.28362
Kim, J. S., & Lee, H. (2009). Inner ear dysfunction due to vertebrobasilar ischemic stroke. Seminars in Neurology, 29(5), 534–540. https://doi.org/10.1055/s-0029-1241037
Kim, J. S., & Kim, H. J. (2012). Inferior vestibular neuritis. Journal of Neurology, 259(8), 1553–1560. https://doi.org/10.1007/s00415-011-6375-4
Lee, S. U., Kim, H. J., Choi, J. Y., & Kim, J. S. (2020). Ictal downbeat nystagmus in Ménière disease: A cross-sectional study. Neurology, 95(17), e2409–e2417. https://doi.org/10.1212/WNL.0000000000010653
Lee, S. U., Kim, H. J., Choi, J. Y., Koo, J. W., Yang, X., & Kim, J. S. (2020). Evolution in the findings of head-impulse tests during the attacks of Menière’s disease. Otology & Neurotology: Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 41(6), e744–e750. https://doi.org/10.1097/MAO.0000000000002645
Luis, L., Costa, J., Vaz Garcia, F., Valls-Solé, J., Brandt, T., & Schneider, E. (2013). Spontaneous plugging of the horizontal semicircular canal with reversible canal dysfunction and recovery of vestibular evoked myogenic potentials. Otology & Neurotology: Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 34(4), 743–747. https://doi.org/10.1097/MAO.0b013e318287f343
Magliulo, G., Gagliardi, S., Ciniglio Appiani, M., Iannella, G., & Re, M. (2014). Vestibular neurolabyrinthitis: a follow-up study with cervical and ocular vestibular evoked myogenic potentials and the video head impulse test. The Annals of Otology, Rhinology, and Laryngology, 123(3), 162–173. https://doi.org/10.1177/0003489414522974
Manzari, L., Burgess, A. M., MacDougall, H. G., Bradshaw, A. P., & Curthoys, I. S. (2011). Rapid fluctuations in dynamic semicircular canal function in early Ménière's disease. European archives of Oto-Rhino-Laryngology: Official Journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): Affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery, 268(4), 637–639. https://doi.org/10.1007/s00405-010-1442-5
Manzari, L., Tedesco, A. R., Burgess, A. M., & Curthoys, I. S. (2010). Ocular and cervical vestibular-evoked myogenic potentials to bone conducted vibration in Ménière's disease during quiescence vs during acute attacks. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 121(7), 1092–1101. https://doi.org/10.1016/j.clinph.2010.02.003
Martinez-Lopez, M., Manrique-Huarte, R., & Perez-Fernandez, N. (2015). A puzzle of vestibular physiology in a Meniere's disease acute attack. Case Reports in Otolaryngology, 2015, 460757. https://doi.org/10.1155/2015/460757
Mazzoni A. (1990). The vascular anatomy of the vestibular labyrinth in man. Acta Oto-Laryngologica. Supplementum, 472, 1–83. https://doi.org/10.3109/00016489009121137
Minor, L. B., Solomon, D., Zinreich, J. S., & Zee, D. S. (1998). Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Archives of Otolaryngology--Head & Neck Surgery, 124(3), 249–258. https://doi.org/10.1001/archotol.124.3.249
Murofushi, T., Tsubota, M., & Suzuki, D. (2019). Idiopathic acute high-tone sensorineural hearing loss accompanied by vertigo: vestibulo-cochlear artery syndrome? Consideration based on VEMP and vHIT. Journal of Neurology, 266(8), 2066–2067. https://doi.org/10.1007/s00415-019-09353-6
Navari, E., & Casani, A. P. (2020). Lesion patterns and possible implications for recovery in acute unilateral vestibulopathy. Otology & Neurotology: Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 41(2), e250–e255. https://doi.org/10.1097/MAO.0000000000002476
Pogson, J. M., Taylor, R. L., Young, A. S., McGarvie, L. A., Flanagan, S., Halmagyi, G. M., & Welgampola, M. S. (2016). Vertigo with sudden hearing loss: audio-vestibular characteristics. Journal of Neurology, 263(10), 2086–2096. https://doi.org/10.1007/s00415-016-8214-0
Rahne, T., Plößl, S., Plontke, S. K., & Strauss, C. (2018). Preoperative determination of nerve of origin in patients with vestibular schwannoma. HNO, 66(Suppl 1), 16–21. https://doi.org/10.1007/s00106-017-0416-y
Rambold, H., Boenki, J., Stritzke, G., Wisst, F., Neppert, B., & Helmchen, C. (2005). Differential vestibular dysfunction in sudden unilateral hearing loss. Neurology, 64(1), 148–151. https://doi.org/10.1212/01.WNL.0000148599.18397.D2
Schuknecht, H. F. (1993). Pathology of the ear (2nd ed.). Philadelphia: Lea & Febiger.
Tarnutzer, A. A., Bockisch, C. J., Buffone, E., & Weber, K. P. (2017). Association of posterior semicircular canal hypofunction on video-head-impulse testing with other vestibulo-cochlear deficits. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 128(8), 1532–1541. https://doi.org/10.1016/j.clinph.2017.04.029
Taylor, R. L., Kong, J., Flanagan, S., Pogson, J., Croxson, G., Pohl, D., & Welgampola, M. S. (2015). Prevalence of vestibular dysfunction in patients with vestibular schwannoma using video head-impulses and vestibular-evoked potentials. Journal of Neurology, 262(5), 1228–1237. https://doi.org/10.1007/s00415-015-7697-4
Vannucchi, P., Pecci, R., Giannoni, B., Di Giustino, F., Santimone, R., & Mengucci, A. (2015). Apogeotropic posterior semicircular canal benign paroxysmal positional vertigo: some clinical and therapeutic considerations. Audiology Research, 5(1), 130. https://doi.org/10.4081/audiores.2015.130
Ward, B. K., Carey, J. P., & Minor, L. B. (2017). Superior canal dehiscence syndrome: Lessons from the first 20 years. Frontiers in Neurology, 8, 177. https://doi.org/10.3389/fneur.2017.00177
Yacovino, D. A., Hain, T. C., & Musazzi, M. (2017). Fluctuating vestibulo-ocular reflex in Ménière's disease. Otology & Neurotology: Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 38(2), 244–247. https://doi.org/10.1097/MAO.0000000000001298
Zulueta-Santos, C., Lujan, B., Manrique-Huarte, R., & Perez-Fernandez, N. (2014). The vestibulo-ocular reflex assessment in patients with Ménière's disease: examining all semicircular canals. Acta Oto-Laryngologica, 134(11), 1128–1133. https://doi.org/10.3109/00016489.2014.919405


