Question
Who is a candidate for the MED-EL SYNCHRONY Electric-Acoustic Stimulation (EAS) Cochlear Implant System?
Answer
With recent FDA approval of the SYNCHRONY Electric-Acoustic Stimulation (EAS) Cochlear Implant System, individuals with high-frequency hearing loss have a new option for an implantable hearing device with positive outcomes demonstrated through a clinical trial with 73 subjects. MED-EL’s EAS System is approved for adult candidates with residual low frequency hearing and severe to profound high-frequency sensorineural hearing loss. Often called “ski-slope” hearing loss, individuals with this configuration of loss are often not able to obtain sufficient benefit from appropriately fit hearing aids. Acoustic hearing aids are frequently unable to provide adequate high-frequency amplification for such severe to profound hearing loss. This results in patients often reporting that they are able to hear speech and other sounds around them but not able to understand what is being said. Understanding speech, which comes from high-frequency sounds (speech sounds such as vowels, as opposed to consonants), can be even more difficult in significant background noise. EAS, which combines a cochlear implant to stimulate high-frequencies with an acoustic component to provide low-frequency amplification, can improve speech understanding both in quiet and in background noise. Participants in the EAS clinical trial demonstrated significant improvements in speech understanding in both conditions, as well as increases in satisfaction and perceived benefit from the device, compared to traditional hearing aids.
Candidacy for the SYNCHRONY EAS System is determined not only by normal hearing to moderate hearing loss in the low frequencies and severe to profound hearing loss in the high frequencies on the audiogram, but also by speech recognition scores. It is important to verify that patients with hearing loss meeting EAS criteria are also not performing satisfactorily with appropriately fit hearing aids on measures of speech understanding in quiet. Candidates for EAS should score 60% or less on CNC words in quiet in each ear in the aided condition.
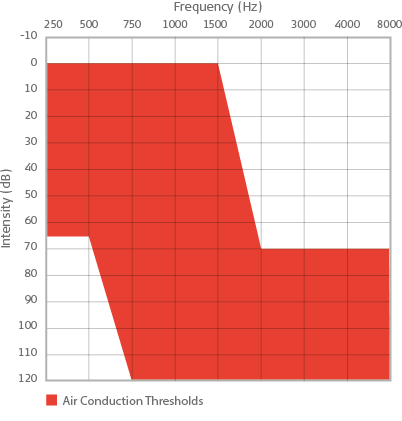
Red area represents unaided air conduction audiometric thresholds for candidacy for the SYNCHRONY EAS Cochlear Implant System.
Based on the results from the clinical trial, a patient who meets both audiometric and speech criteria noted above is likely to experience improvements in speech understanding in quiet and in noise, as well as an increase in self-perceived benefit from the device. Individuals with this configuration of hearing loss have often relied for many years on what low-frequency hearing they do have and may be reluctant to put this hearing at risk. The risk-benefit profile of all available options should be considered when counseling patients with ski-slope hearing loss. The risk of loss of residual hearing can be minimized through surgical technique and electrode design, and outcomes from the clinical trial or other experience with MED-EL’s EAS System should be discussed with potential candidates to determine if EAS implantation is a viable option.
For more information, visit www.medel.com/us or the MED-EL Expo on AudiologyOnline.

