Editor’s Note: This text course is a transcript of a live, expert seminar on AudiologyOnline. Download supplemental course materials here.
Robert Battista: Today we will provide an overview about acoustic neuromas. Subsequent lectures from members of our practice will cover more specific aspects of acoustic neuroma and surgical treatment, but today we will give you broad overview of the topic. An acoustic neuroma is a benign, often slow-growing intracranial tumor. These originate off of the Schwann cells of the vestibular nerve. Acoustic neuroma is the most common term, but the more accurate term is vestibular schwannoma because these tumors often arise off the vestibular portion of the VIIIth cranial nerve. The VIIIth cranial nerve has two components: auditory and vestibular. For discussion purposes today, we will use the term acoustic neuroma because it is the most widely used and recognized.
Acoustic neuromas account for six to eight percent of intracranial tumors. The incidence is approximately 10 to 20 per 1,000,000 people in the United States (Nestor, Korol, Nutik, & Smith, 1988; Stangerup, Tos, Caye-Thomasen, Tos, Klokker, & Thomasen., 2004). This translates to between 2,500 and 3,500 new cases per year. In our Chicago practice, we see between 10 and 20 cases of acoustic neuroma per month for one reason or another.
There are broad types of acoustic neuroma: sporadic and neurofibromatosis 2 (NF2). Sporadic tumors make up the vast majority or 95% of all cases. NF2, accounts for 5% of acoustic neuromas, and the vast majority of patients with NF2 have bilateral acoustic neuromas with a single solitary lesion on each VIIIth nerve. These tumors can come in various sizes and shapes, which will be shown in a moment. A review of the anatomy will be helpful as we discuss and look at different areas of the auditory pathway.
Figure 1 is a cross-section of the brain from an axial view. This view looks down on the internal auditory canal and seventh and VIIIth cranial nerves as they pass through. The VIIIth cranial nerve starts at the brainstem and runs a 2-cm course through the bony tunnel to the inner ear. Acoustic neuromas most commonly arise at the junction of the internal auditory canal.
Figure 1. View PDF
Figure 2 shows this same axial cross-section of the brain, but with a small intracanalicular acoustic neuroma that fills the internal auditory canal. These tumors will often start in this canal, and they can stay there undetected or undisturbed for many years. We have patients in our practice that we have been following for a number of years where there has been little to no growth within the canal. When they move out of the canal, however, there is typically accelerated growth because it is an open space relative to a small, bony tunnel.
Figure 2. View PDF
Hearing and balance symptoms are the most common presenting symptoms for these patients. Naturally, as the tumor enlarges the symptoms change. Hearing worsens, symptoms of disequilibrium worsen, and tinnitus becomes louder.
Figure 3 is a medium-size tumor impinging the boundary of brainstem, and there may be slight compression of the brainstem as well. Because these tumors typically grow slowly there is a very little in the way of unsteadiness at this point in time with this size tumor.
Figure 3. View PDF
By contrast again, Figure 4 shows a large tumor. This is not only compressing the brainstem, which can cause problems with breathing and central disequilibrium because the cerebellum is compressed, but the Vth cranial nerve, which controls sensation to the face, is affected. So along with hearing loss and disequilibrium, patients may experience difficulties breathing and numbness of the face if the tumors grow too large.
Figure 4. View PDF
Signs and Symptoms
Unilateral sensorineural hearing loss is most common presenting symptom of a patient with an acoustic neuroma. The second most common symptom is that of unilateral tinnitus. Because of this, anyone who has unilateral sensorineural hearing loss that is unexplained, in our opinion, has an acoustic neuroma until proven otherwise. To give you example of this, I saw a patient today who has a hearing loss in one ear and previously saw an audiologist who found a left-sided moderate-to-severe mid-to-high frequency sensorineural hearing loss. The patient was fitted with a hearing aid but a year later still wondered what was going on, so the patient sought the advice of a physician. That physician ordered an MRI, and this patient, sure enough, has small acoustic neuroma on the left side.
Disequilibrium is the third most common symptom for acoustic neuroma patients and happens in 50% of patients. Smaller tumors usually do not cause complete disequilibrium; they cause vertigo. Smaller tumors are an irritation of the vestibular nerve. The reason why only 50% of patients report vertigo is that the tumors grow so slowly, thus giving the patient time to compensate for small changes in vestibular function. They do this without noticing and experience very little in the way dizziness or disequilibrium initially. As the tumor enlarges, disequilibrium can occur. Five to 20% of patients will experience facial numbness and less than 5% will experience facial twitching. Headaches are not a common symptom until the tumors get fairly large.
Diagnosis
Once we suspect acoustic neuroma, there are tools we use to confirm or rule out that diagnosis. The audiogram is screening method; so is auditory brainstem response (ABR) testing. Magnetic resonance imaging (MRI) is the gold standard of diagnosing an acoustic neuroma. It is important to be reminded that MRIs can show false-positive findings. When we order these MRIs, we always request that they performed with gadolinium, which is a dye that will light up the tumors on the MRI. These tumors are typically very obvious. The smallest tumor that can be identified is 2 millimeters in size. We do not rush to begin treatment on any lesions around 2 to 3 millimeters in size, because that can sometimes be inflammation and not an actual tumor. We get a scan again at six to nine months later to see if there is any change. If the spot was inflammation, we will likely see this decrease in size over time.
What about the hearing loss you see in these patients? The vast majority of patients experience slowly progressive, unilateral sensorineural hearing loss. Only 10% of patients with acoustic neuromas present with sudden hearing loss. As we know, steroids are something that can treat sudden sensorineural hearing loss, and I personally have had a few patients whose sudden hearing loss improved with the use of steroids but still have an acoustic neuroma. Just because a patient's hearing has improved with steroids it does not mean a tumor has been ruled out. There is still a need for an MRI on patients that have had a unilateral sensorineural hearing loss, even if hearing improved with steroids.
To break it down further, what type of hearing loss do these patients have? Two-thirds have high-frequency sensorineural hearing loss similar to the cases mentioned earlier. About one-third will have low-frequency or mid-frequency loss, and as many as 12% of patients can have normal hearing in the presence of an acoustic neuroma. Figure 5 shows real audiometric data of someone with a right-sided acoustic neuroma. This person has very subtle hearing difference between ears, but the person complains of unilateral tinnitus on the right side. That was the tipoff, along with the audiogram, to get the MRI to make the diagnosis.
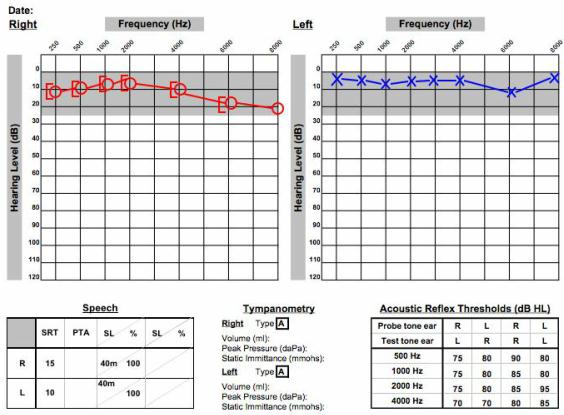
Figure 5. Audiometrical data of a patient with a unilateral acoustic neuroma on the right side.
When the MRI was done, a large tumor was found on the right side. The gadolinium lit up the tumor. There were parts within the mass that did not illuminate with the gadolinium which indicated fluid or blood inside the tumor. This was a person with normal hearing, but there it was. You always have to keep this in the back of your mind for a patient with these symptoms of unilateral tinnitus and slight asymmetries in the hearing. Any type of unilateral symptoms could be acoustic neuroma.
Treatments
Treatment, for purposes of this course, is broken down into three categories: observation, stereotactic radiosurgery and microsurgical removal. We will discuss these briefly, but know that there will be further webinars on this topic. Treatment by observation means that we obtain serial MRI scans for those patients who are relatively asymptomatic and over the age of 65. This is not a hard-and-fast rule, but rather a guideline; each case must be considered on an individual basis.
Patients under observation will have a scan about six months after the initial MRI. If there is no change in the size of the tumor, then we can get another scan about nine months later and then follow-up with yearly scans after that. This approach is for tumors that are less than two- to two-and-a-half centimeters in size. If tumors are larger than two-and-a-half centimeters in size, we do not observe the patients because brainstem compression is a real possibility. With this size and an increased number of symptoms we want to treat with either radiosurgery or micro surgery. A patient would be moved to radiosurgery or the microsurgical arm of treatment when symptoms change dramatically and there is a significant change in the size of the tumor.
Stereotactic radiosurgery was used for the elderly who were experiencing significant symptoms. It is a good treatment in select situations, whether they have good medical status or poor medical status, but only for tumors less than three centimeters in size. We do not recommend radiosurgery for tumors larger than three centimeters because there is too much radiation given to the surrounding structures and too much in the way of side effects. Sometimes the tumor is large enough that there is adherence of the tumor to the facial nerve, and it is very difficult to remove the tumor off the facial nerve. In those cases, we may have to leave a little bit of the tumor and then monitor the residual tumor. Sometimes the tumors will not grow, but if they do they are good candidates for radiosurgery follow-up after a microsurgery.
Microsurgery is reserved for all other cases not mentioned here, but there are some people, no matter the size of the tumor, who would feel more comfortable if the tumor were completely removed, and we can do that the vast majority of time in microsurgery.
There are several considerations for the three different management options. First is the patient’s age. If the person is elderly or frail, it may be wisest to observe the tumor if it is small. If the tumors were to enlarge slightly we might recommend radiosurgery. We will not know the growth rate unless we have more than one scan. The health status of the patient must be taken into account. If they have a vast array of medical problems, we do not want to operate on those patients. The size of the tumor is a weighty consideration. We must look at both the symptoms the patient is experiencing and the size of the tumor. If someone is having disabling dizziness as a prime symptom, we usually do not recommend observation or radiosurgery, but rather microsurgery. Microsurgery gives a much better chance of eliminating the person's dizziness or improving the disequilibrium as opposed to radiosurgery.
Risks
There are inherent risks involved with any of these three treatment options. One of the risks of observation is hearing loss. Even if the tumor does not change in size by MRI tests, we have seen hearing deteriorate. It is hypothesized that this is due to changes in blood flow to the acoustic neuroma, and some things may be happening on a microscopic level that the MRI does not pick up. In addition, even if the tumor does not grow, some people may start having dizziness symptoms. We are not sure why that happens but it may be due to changes in blood flow as well.
If the growing tumor were to result in brainstem compression, we would not recommend observation. We recommend either radiosurgery or microsurgery depending on the size. Stereotactic radiosurgery was started in the early 1950s by a Swedish physician, and is now what we know as Gamma Knife radiosurgery. The other way of delivering radiation is called CyberKnife radiosurgery. Gamma Knife gives more radiation than CyberKnife, and as such, CyberKnife is becoming more popular. Both of these techniques are relatively simple from the patient's standpoint.
Gamma Knife radiosurgery is a single session treatment. The patient can have a morning visit to the hospital and often go back to work the next day. This is a very attractive option to patients. It is important to point out that with radiosurgery the tumor is not removed. The tumor is controlled. This means that we are trying to prevent the tumor from causing symptoms. In some cases, the tumor does get smaller with radiosurgery, but the vast majority of tumors are controlled where they do not get bigger.
With Gamma Knife, there are 201 gamma rays delivered to the tumor. Each ray, in and of itself, is relatively harmless. The beams are able to penetrate just at the tumor level to surround the tumor so the radiation is isolated to the specific area. There is very little spill-over of the radiation to the surrounding structures. CyberKnife is usually done over a three-session visit, but with the same principle that radiation is delivered very specifically to the tumor.
Surgical approaches have changed dramatically since the early 20th century. In the early 1900's, the doctor used to open the skull and pull the tumor out with their finger. Thank goodness things have come a long way since then. Microsurgery is now done with three basic techniques: translabyrinthine, retrosigmoid and middle fossa. The translabyrinthine approach (Figure 6) is taken through the inner ear.
Figure 6. View PDF
The incision is made behind the ear, where the labyrinth bone is then exposed. The facial nerve must be identified within the cavity before we start the dissection of the tumor. In Figure 6, there is a bit of compression of the brain. In these cases, the neurotologist will work with a neurosurgeon to remove the tumors out. The job of the neurotologist is to expose the area and remove the portion of the tumor within the area of the inner ear, and the neurosurgeon takes out the rest of the tumor near the brain.
The retrosigmoid approach is taken behind the sigmoid sinus. It uses the same type of incision behind the ear but the bone behind the sigmoid sinus is opened. The bone of the internal auditory canal is opened to expose the canal, skirting along the labyrinth. We use this technique when preservation of hearing is a key component. With this technique, we remove the tumor before we locate the facial nerve. The nerve is located on the anterior edge of the tumor here, so the tumor has to be removed before we can see the facial nerve.
The third approach is the middle fossa approach (Figure 7). In this approach, we also go around the labyrinth to try to preserve hearing. We open up the bone above the ear, compressing the brain slightly. We remove the bone to get to the tumor from above rather from behind like the other approaches. The middle fossa approach is only for tumors that are in the internal auditory canal. Sometimes the tumors may spill out from the internal auditory canal, but we cannot operate much past the internal auditory canal with the middle fossa approach.
Figure 7. View PDF
In the translabyrinthine approach all hearing is lost, so this approach is reserved for patients who already have poor hearing or for people that we feel we have a very low chance of preserving hearing. The potential for hearing preservation occurs with the middle fossa or retrosigmoid approaches. It is not always possible, but we certainly try.
There are, in fact, pre-operative prognostic signs indicating that we might be able to preserve hearing with either the retrosigmoid or middle fossa approach. First, the acoustic neuroma needs to be less than one-half centimeters in size. The audiogram needs to show better than a 50 dB pure-tone average and better than 50% word recognition score. Ideally, the ABR should be normal, the caloric response should be reduced on the side of the tumor, and the VEMP should be within normal limits.
Figure 8 shows a cross-section through the internal auditory canal. The cross section includes the superior vestibular nerve, inferior vestibular nerve, VIIth cranial nerve or the facial nerve, and the cochlear nerve.
Figure 8. View PDF
Caloric testing measures the superior vestibular nerve through the lateral semi-circular canal, and the VEMP tests the inferior vestibular nerve. A large portion of these tumors will start on the superior vestibular nerve, and as they enlarge they will compress the cochlear nerve. If the tumors start on the inferior vestibular nerve they are much closer and contiguous with the cochlear nerve, so there is less chance of hearing preservation when removing these types of tumors. There is no real way to know this based on the MRI, so we rely heavily on these preoperative tests.
When the superior vestibular nerve is affected by the tumor (Figure 9), we see a slight compression of the facial nerve.
Figure 9. View PDF
The facial nerve is very resilient, so we see very few symptoms in terms of facial nerve conditions preoperatively. Out of the four nerves on the cross-section, the cochlear nerve is the most sensitive, so when it is compressed even slightly we see symptoms of hearing loss. In these cases (Figure 9) there is reduced or caloric response because the tumor affects the superior vestibular nerve, and the VEMP is normal because the inferior vestibular nerve is still functioning.
The opposite is true for inferior vestibular nerve tumors (Figure 10). The caloric response is normal because the superior vestibular nerve is still functioning, and the VEMP is reduced or absent because the tumor is through the inferior vestibular nerve. This situation has a poor prognosis with regard to hearing because it affects the cochlear nerve so closely. We take these things into consideration when contemplating surgery.
Figure 10. View PDF
Audiological Aspects of Acoustic Neuroma
Jill Messina: I will talk about the audiological aspects that aid the diagnosis and prognosis for a patient with acoustic neuroma. As far as conventional audiometry is concerned, the most common abnormality we see is an asymmetric, high-frequency hearing loss on the side affected by the acoustic neuroma. Dr. Battista talked a little bit about the typical audiological configuration being high frequency, but about a third of cases can be low-frequency or mid-frequency hearing loss or even normal hearing.
Figure 11 is an audiogram that depicts the typical high-frequency sensorineural hearing loss that we might see. This is a 51-year-old female who was diagnosed with acoustic neuroma. She presented to the clinic with a complaint of left-ear hearing loss and tinnitus. Her word recognition score on the left side is still good, which is a positive factor for her. Keep in mind that people can present with normal hearing or symmetric hearing and still have acoustic neuroma. It is important to have a detailed case history and report of the patient's symptoms so that we have the most information with which to correctly identify these patients.
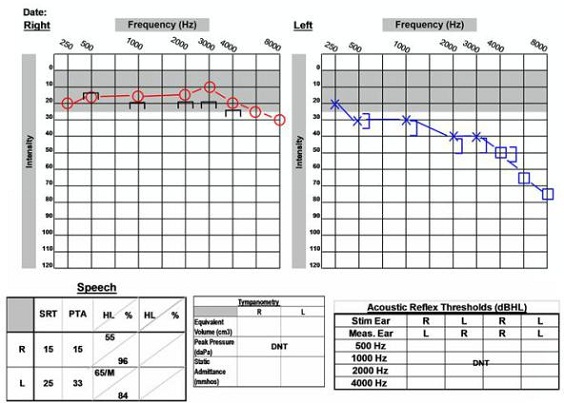
Figure 11. 51-year old with asymmetrical audiogram and acoustic neuroma on the left side.
This patient (Figure 12) has normal, symmetric hearing. The patient has excellent word recognition scores and present acoustic reflexes. This is a 61-year-old female who was diagnosed with acoustic neuroma on the right side. She presented to the clinic with complaints of dizziness, accompanied by right-sided pressure and pain. She also reported bilateral hyperacusis and constant head pressure.
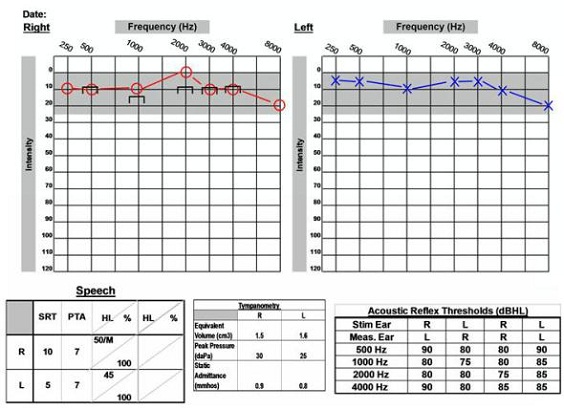
Figure 12. 61-year old with normal audiogram and acoustic neuroma on the right side.
Another important aspect of the audiogram is acoustic reflexes. An audiologist may see absent or elevated responses with an acoustic neuroma. Keep in mind, however, that this is not the most sensitive measure. We saw on the previous audiogram (Figure 11) that reflexes may still be within normal limits in patients with acoustic neuromas. Patients may also have absent reflexes for other reasons, so we cannot rely on this measure alone.
We also want to look at a patient's speech discrimination. It may often be worse than we would expect based on the audiogram. The patient may present with mild or moderate hearing loss, but have poor word understanding that we would not expect otherwise. This can be associated with an acoustic neuroma. Also, we want to look for rollover, which is the decreased ability to understand words when the presentation level is increased.
ABR Testing
ABR testing is less sensitive than the MRI as Dr. Battista said, but is less expensive than the MRI. It can be important in the follow-up care of the patient. A characteristic finding on the ABR is a present wave I but absent waves III or V. You may also see a delayed I-III absolute latency or a delayed wave V latency. It is also important to pay attention to the differences between ears and look for any significant interaural differences, because that can indicate possible acoustic neuroma.. Keep in mind that there are high false-positive and false-negative rates associated with the ABR. Patients with small tumors can have normal ABRs. The ABR is more accurate the larger the tumor is, so it has 92 to 98% accuracy when the acoustic neuroma is greater than one-and-a-half centimeters, but as it gets smaller than that, the accuracy drops to 60 to 70% (Schmidt, Sataloff, Newman, Spiegel, & Myers, 2001). Even if a patient has a normal ABR, an acoustic neuroma cannot be ruled out.
Figure13 shows the ABR tracings for a patient of ours. She was diagnosed with left-side acoustic neuroma. We can see that waves I, III and V are clearly identified in the right ear. Morphology should be very clear and appropriate, easy identifiable and repeatable. We want to use slow and fast rates to compare latency shift with an increased rate. If the shift is too delayed, then that can be a sign of acoustic neuroma. Also measure the absolute and the inter-peak latencies. The tracings for the left ear are, in comparison, much poorer than the right ear. We can only identify wave I, and waves III and V are absent.
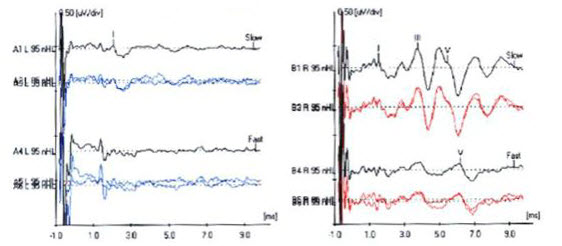
Figure 13. ABR tracings of patient with acoustic neuroma on the left side. Note left-ear diminished wave I and absent waves III and V.
Electronystagmography and videonystagmography (ENG/VNG) is another tool that can give us information about acoustic neuroma. Many patients will have a unilateral caloric weakness on ENG or VNG testing. Caloric weaknesses, however, can be caused by other conditions, so it is not a specific test. Vestibular-evoked myogenic potential (VEMP) testing is something we would want to complete which provides insight into an acoustic neuroma that may exist on the inferior vestibular nerve. VEMPs tend to be absent in approximately 80% of patients with acoustic neuroma (Murofushi, Matsuzaki, & Mizuno, 1998).
Case Studies
These are interesting cases of patients that have been seen in our clinic. This first case is of a 16-year-old male who came to the clinic, complaining of right-sided hearing loss and vertigo. He also noted that about a year before coming to the clinic he had had some right-ear pressure or fullness. He visited his primary care physician about six months before seeing us and was found to have fluid in the right middle ear space. That was treated and the fluid resolved, but he still noticed a hearing loss in the right ear, so he sought out further evaluation. The audiogram indicates a mild low-frequency sensorineural hearing loss on the right side (Figure 14), and with absent acoustic reflexes in the right ear, but excellent word recognition scores bilaterally. The VNG yielded a unilateral weakness. This patient had a four-and-a-half centimeter acoustic neuroma on the right side, and this was removed with the translabyrinthine procedure. Due to the patient's young age, he was also tested for neurofibromatosis 2 (NF2), which came back positive. If other tumors arise for patients that are young, doctors typically do testing to see if NF2 is a possibility.
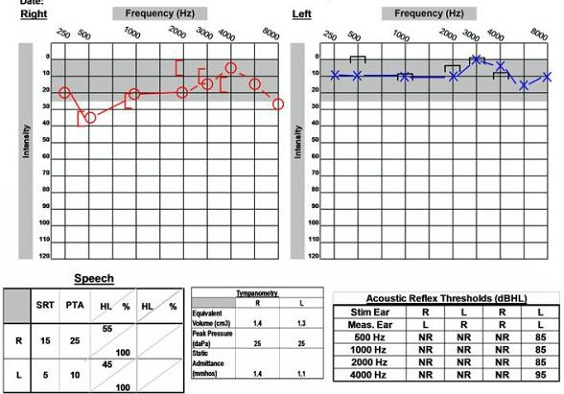
Figure 14. Audiological data for 16-year old with NF2 and acoustic neuroma on the right side.
The second case study is a 59-year-old male who came to the clinic reporting left-side tinnitus and vertigo. The MRI shows a small acoustic neuroma measuring 1.1 centimeters. Figure 15 shows this patient’s audiogram. You can see a high-frequency sensorineural hearing loss in the left ear and a word record recognition score of 84%, so his understanding is still good in that ear. Based on the degree of the hearing loss, the good word recognition score and the small size of the tumor, this looks like a case where hearing may be preserved. When we look at the other diagnostic testing, he had an abnormal ABR with prolonged latencies and a normal VNG. Those results are poor indicators for hearing preservation. The patient and physician decided to proceed with a retrosigmoid surgical approach to remove the tumor. The patient had some positive indicators related to the audiological hearing loss, but some poor indicators with ABR and VNG testing.
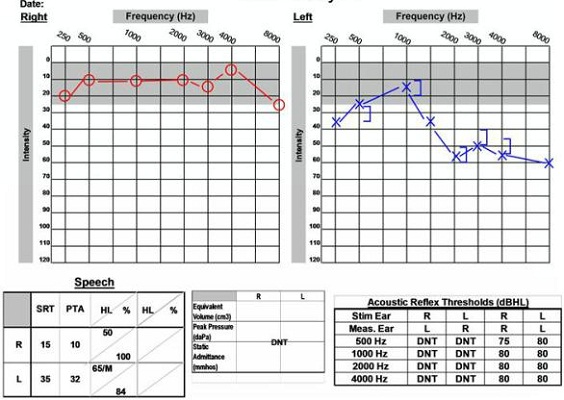
Figure 15. Audiological data for a 59-year old male with acoustic neuroma on the left side, accompanied by abnormal ABR and normal VEMP.
As shown in the post-operative audiogram (Figure 16), hearing was able to be preserved. The pre-operative hearing loss in that left ear persists, but his word recognition score is still 76%. This indicates that this patient could benefit from amplification on that side and have a positive outcome.
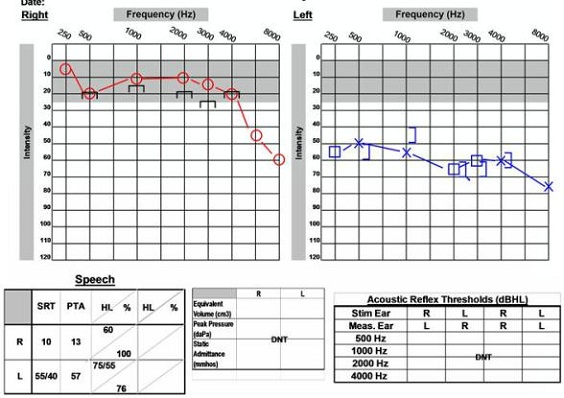
Figure 16. Post-operative audiological data of 59-year old male, with preservation of word recognition abilities.
The last case is of a 58-year-old female. She came in with complaints of sudden right-ear hearing loss, tinnitus on the right side and dizziness. She reported that she had a similar incident on the left side about 20 years earlier. Figure 17 is her audiogram on the day she came into the clinic. She reported that she awoke with loud ringing in the right ear and became dizzy, and that the hearing loss happened suddenly. She had moderately severe to severe hearing loss in the right ear and severe hearing loss in the left ear with no word recognition ability on either side, so it was recommended that she have a steroid injection on the right side and undergo an MRI. If hearing did not improve after the steroid treatment, then she was going to consider a cochlear implant evaluation.
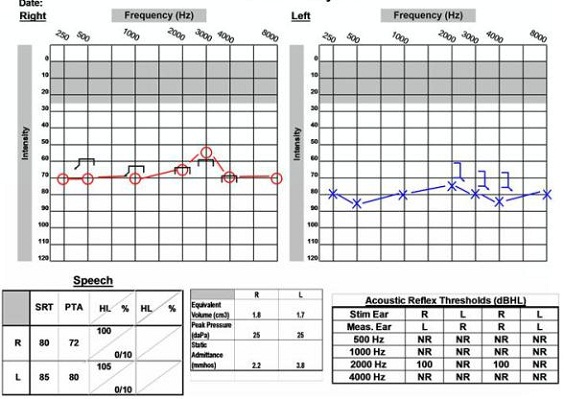
Figure 17. Audiological data of 58-year old female with sudden hearing loss and right acoustic neuroma.
This patient completed the MRI, which revealed a small acoustic neuroma on the left side. This was the side on which she originally experienced the sudden hearing loss 20 years ago. That was something unexpected when doing the MRI, as we expected to see something on the right ear instead. It added another dimension to the case. This patient's hearing in the right ear did not recover, so she was interested in proceeding with the cochlear implant.
We had to consider the options, which included what side would be implanted since she would likely be having serial MRIs completed to monitor the acoustic neuroma on the left side. The magnet of the cochlear implant would have to be removed each time the patient underwent an MRI. Another option was to have the acoustic neuroma removed on the left side and to proceed with a cochlear implant on the right side. She did decide she wanted to have the acoustic neuroma removed from the left ear, and that was completed with the translabyrinthine procedure. Approximately two months later she was implanted with a cochlear implant on the right side. Figure 18 is her post-operative audiogram which shows that she is doing fairly well with her cochlear implant.
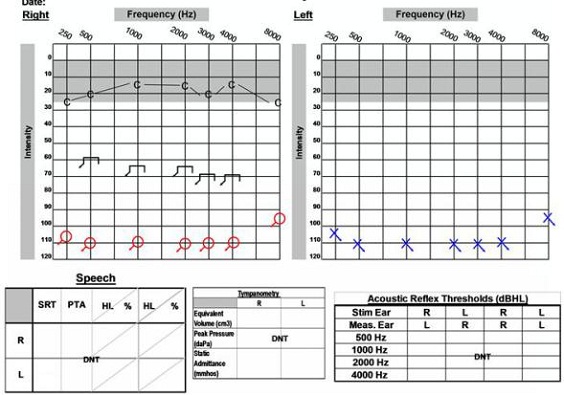
Figure 18. Post-operative audiogram of 58-year old female with left acoustic neuroma on the right side, which was removed and post cochlear implantation on the right ear. C= cochlear implant thresholds.
That completes our audiological case studies as they relates to the acoustic neuroma, so now we would be happy to take any questions.
Question & Answer
There is quite a bit of variability in audiological testing to identify retrocochlear tumors. Do you find acoustic decay rollover useful?
Messina: Yes. I would say we always complete it, even in patients where we are seeing normal hearing. It is standard protocol within our clinic to complete acoustic reflexes and decay testing. However, we have seen patients with acoustic neuroma where those tests do come back normal, so they always follow up with the doctor and look at the whole case history. If there is a strong suspicion of neuroma, an MRI is almost always performed.
Do you recommend intraoperative monitoring during surgery for cranial nerves that could be affected by surgery, and is this dependent on what surgical approach is used?
Battista: That is a good question. During surgery we do facial nerve monitoring on all approaches. Intraoperative auditory monitoring is performed for the middle fossa and retrosigmoid cases where we are trying to preserve hearing. That could be done a number of ways. It could be direct VIIIth nerve monitoring, ECoG monitoring, or ABR monitoring. We have done all three. One approach does not seem to be better than the other. We use ABR monitoring frequently during the case as we are looking at potential hearing preservation.
You mentioned that VEMP typically has decreased amplitude. Can you define what you mean by “decreased” and what amplitude range you are using?
Messina: Our clinical cut-off for normal amplitude in the VEMP is 60 microvolts.
What do you consider fast and slow click rates?
Messina: Good question. We use 11.1 clicks per second for slow and 91.1 clicks per second for fast. We look to see if the wave V shift is less than 0.8 milliseconds between click rates.
What is the average growth rate of the acoustic tumor, and how often do you see patients where the tumor does not grow at all?
Battista: There are over 100 patients in our practice that we have been following for a number of years with little to no growth. We consider any growth greater than 2 millimeters compared to the previous scan significant. However, we also have to look at the original scan as well. Sometimes, when doing scans over a number of years, we do not see a significant change per visit, but when compared to the original scan the tumor has increased significantly. We would likely recommend treatment in those cases. There are a percentage of tumors that do not grow. No one knows for sure the cause in these cases. It all depends on the size and location of the tumor. Quite frankly, I cannot give a blanket statement about that, but if the tumors are inside the internal auditory canal, many of the tumors will not grow when you follow them.
Is it routine to perform a follow-up VEMP test after surgery to look at the integrity of the inferior nerve?
Battista: Good question. We do not typically do VEMP post-operatively in our practice. We are doing VEMP preoperatively for prognostic reasons.
What are the OAE findings that you would expect with acoustic neuroma?
Messina: I have seen robust OAEs in the presence of acoustic neuroma because the OAEs arise from the cochlea, not the nerve. If hearing loss associated, you may not see them, but if the hearing loss is present secondary to the neuroma, then the hair cells in the inner ear are still able to contribute to the presence of OAEs.
References
Jackler, R.J. (2005). The atlas of skull base surgery and neurotology (first edition). NY: Thieme.
Murofushi, T., Matsuzaki, M., & Mizuno, M. (1998). Vestibular evoked myogenic potentials in patients with acoustic neuromas. Archives of Otolaryngology- Head & Neck Surgery, 124, 509-512.
Nestor, J.J., Korol, H.W., Nutik, S.L., & Smith, R. (1988). The incidence of acoustic neuromas. Archives of Otolaryngology- Head & Neck Surgery, 114(6), 680.
Schmidt, R., Sataloff, R.T., Newman, J., Spiegel, J.R., & Myers, D.L. (2001). The sensitivity of auditory brainstem response testing for the diagnosis of acoustic neuromas. Archives of Otolaryngology- Head & Neck Surgery, 127, 19-22.
Stangerup, S.E., Tos, M., Caye-Thomasen, P., Tos, T., Klokker, M., & Thomsen, J. (2004). Increasing annual incidence of vestibular schwannoma and age at diagnosis. The Journal of Laryngology and Otology, 118(8), 622-627.



